A Probable Case of Tirzepatide-Induced Acute Pancreatitis
- PMID: 40612832
- PMCID: PMC12225653
- DOI: 10.7759/cureus.85291
A Probable Case of Tirzepatide-Induced Acute Pancreatitis
Abstract
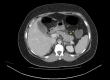
Glucagon-like peptide-1 (GLP-1) receptor agonists have become increasingly popular for their dual benefits in glycaemic control and weight reduction. However, emerging concerns have been raised regarding their association with acute pancreatitis. Tirzepatide, a novel dual GLP-1/gastric inhibitory polypeptide (GIP) receptor agonist, is currently a subject of concern. We report the case of a 32-year-old woman with a prior history of gestational diabetes mellitus, who presented with a three-week history of worsening epigastric pain, nausea, vomiting, and constipation. The patient had commenced tirzepatide (Mounjaro) five weeks earlier for weight loss, with five doses administered prior to admission. She had received 2.5 mg subcutaneously weekly for four weeks, followed by one dose of 5 mg subcutaneously. Examination revealed severe epigastric tenderness without peritonism. Laboratory tests showed markedly elevated lipase (11,645 U/L), transaminases, alkaline phosphatase, and bilirubin levels. Imaging findings were consistent with acute interstitial oedematous pancreatitis and revealed incidental gallstones without signs of choledocholithiasis or cholecystitis. CT and magnetic resonance cholangio-pancreatography (MRCP) confirmed pancreatic inflammation without biliary obstruction. The patient was managed conservatively with cessation of tirzepatide, supportive care, and close monitoring. She showed significant clinical improvement with normalisation of lipase levels by discharge. This case raises concerns over the potential risk of pancreatitis associated with tirzepatide use. Although the patient's pancreatitis may appear to be most consistent with gallstone-induced aetiology, the recent initiation of tirzepatide may be a potential contributing factor, as the strong temporal correlation between drug initiation and symptom onset, coupled with clinical resolution upon discontinuation, suggests a probable causal relationship. Clinicians should maintain a high index of suspicion for drug-induced pancreatitis in patients using GLP-1 receptor agonists, including tirzepatide, especially in those with pre-existing metabolic risk factors or gallstones. This case underscores the need for continued surveillance and more robust data to guide the safe use of tirzepatide in routine clinical practice.
Keywords: acute pancreatits; glp-1 receptor agonist; medication-induced pancreatitis; multiple gallstones; tirzepatide.
Copyright © 2025, Sumaruth et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Nauck MA, D'Alessio DA. https://cardiab.biomedcentral.com/articles/10.1186/s12933-022-01604-7. Cardiovasc Diabetol. 2022;21:169. - PMC - PubMed
-
- Farzam K, Patel P. StatPearls. Treasure Island, Florida: StatPearls Publishing; 2024. Tirzepatide.
-
- Achievement of glycaemic targets with weight loss and without hypoglycaemia in type 2 diabetes with the once-weekly glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide: a post hoc analysis of the SURPASS-1 to -5 studies. Lingvay I, Cheng AY, Levine JA, et al. https://pubmed.ncbi.nlm.nih.gov/36514843/ Diabetes Obes Metab. 2023;25:965–974. - PubMed
-
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2019. Adverse drug reaction probability scale (Naranjo) in drug induced liver injury. - PubMed
Publication types
LinkOut - more resources
Full Text Sources