The fibrin-derived peptide FX06 protects human pulmonary endothelial cells against the COVID-19-triggered cytokine storm
- PMID: 40612940
- PMCID: PMC12225545
- DOI: 10.3389/fimmu.2025.1591860
The fibrin-derived peptide FX06 protects human pulmonary endothelial cells against the COVID-19-triggered cytokine storm
Abstract
Introduction: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a major health emergency since its emergence in late 2019. Endothelial dysfunction is a hallmark of COVID-19, leading to severe illness, i.e. coagulopathy, multi-organ failure. FX06, a fibrin-derived peptide naturally occurring in the human body, formerly known as Bβ15-42, is a promising therapeutic candidate for endothelial complications like capillary leakage in COVID-19 and other forms of acute respiratory disorders. The aim of this project is to investigate whether FX06 can attenuate COVID-19 cytokine-triggered inflammatory processes in vitro.
Methods: To mimic the inflammatory status of COVID-19, a human pulmonary microvascular endothelial cell line (ECs) - HULEC-5a, was treated with a cytokine cocktail comprised of ten different cytokines or chemokines at concentrations found in serum profiles of COVID-19 patients with severe illness, further referred to as the severe cytokine cocktail. ECs were treated with the severe cytokine cocktail for 24 h, in the absence or presence of FX06 for 2 h.
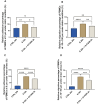
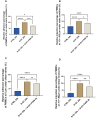
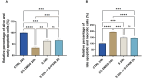
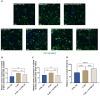
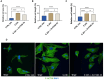
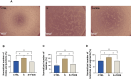
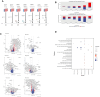
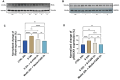
Results: The severe cytokine cocktail enhanced peripheral blood mononuclear cell (PBMC)-endothelial adhesion and monolayer transmigration. This deleterious effect was significantly reduced by FX06. FX06 was also shown to mitigate the cytotoxic activity of allogeneic CD8+ T cells, which increased upon cytokine treatment. FX06 restored continuous vascular endothelial (VE)-cadherin/CD144 distribution on the EC surface and reversed morphological changes mediated by the severe cytokine cocktail, such as the elongation of F-actin stress fibers. FX06 reduced capillary-like structure formation of the severe cytokine cocktail treated-ECs, indicating FX06 down-regulated the pro-inflammatory angiogenic activity caused by the severe cytokine cocktail. Additionally, FX06 might assist in maintaining the normal barrier function of ECs by altering the surface expression of Syndecan-1 (SDC1/CD138). Proteomics and phosphoproteomics analyses demonstrated that FX06 in the presence of the severe cytokine cocktail inactivated RhoGTPase, which was confirmed by western blotting that FX06 attenuated RhoA, a member of RhoGTPase, enhanced by the severe cytokine cocktail and down-regulated the expression of the phosphorylated downstream protein, ROCK1.
Conclusion: Overall, FX06 shows promising potential in normalizing ECs and reducing vascular leakage to protect the endothelium against the proinflammatory effect of COVID-19-triggered cytokines.
Keywords: ARDS; COVID-19; FX06; cytokine; endothelial dysfunction; vascular leakage.
Copyright © 2025 Wang, Lebedev, Li, Rao, Wu, Doyle, Wynne, Blanco, Mysior, Simpson, Scholz, Wülfroth, Zacharowski, Kolch, Zhernovkov and Eissner.
Conflict of interest statement
PW owns shares and is an employee of F4 Pharma, the developer of FX06. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

