Flippase-Mediated Hybrid Vesicle Division
- PMID: 40613217
- PMCID: PMC12506619
- DOI: 10.1002/adma.202504519
Flippase-Mediated Hybrid Vesicle Division
Abstract
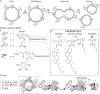
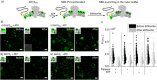
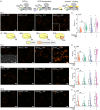
The assembly of synthetic systems with the ability for protein-mediated division remains a challenge in bottom-up synthetic biology. Here, the reconstitution of an active Drs2p-Cdc50p lipid flippase in polymer lipid hybrid vesicles (HVs) made from phospholipids and 1 or 2.5 mol% amphiphilic block copolymers, with poly(carboxyethyl acrylate) or poly(6-O-methacryloyl-d-galactopyranose) as the hydrophilic extension and either cholesteryl methacrylate or butyl methacrylate or combinations thereof as the hydrophobic blocks is demonstrated. The reconstitution of Drs2p-Cdc50p in HVs flip 2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) lipids from the inner to the outer leaflet, leading to transmembrane asymmetry. Importantly, the chemical nature of the hydrophobic block in the amphiphilic block copolymers used to assemble the HVs is crucial to support changes in the spontaneous curvature of the bilayers due to translocation of DOPS lipids that results in HV constriction and division. Taken together, this effort is a step forward in imitating cell division in synthetic assemblies toward potentially bottom-up assembled self-replicating units.
Keywords: hybrid vesicle; lipid flippase; membrane constriction; transmembrane asymmetry; vesicle division.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Schwille P., Spatz J., Landfester K., Bodenschatz E., Herminghaus S., Sourjik V., Erb T. J., Bastiaens P., Lipowsky R., Hyman A., Dabrock P., Baret J.‐C., Vidakovic‐Koch T., Bieling P., Dimova R., Mutschler H., Robinson T., Tang T.‐Y. D., Wegner S., Sundmacher K., Angew. Chem., Int. Ed. 2018, 57, 13382; - PubMed
- b) Mulla Y., Aufderhorst‐Roberts A., Koenderink G. H., Phys. Biol. 2018, 15, 041001; - PubMed
- c) Stano P., Life 2019, 9, 3.
-
- Chang M.‐Y., Ariyama H., Huck W. T. S., Deng N.‐N., Chem. Soc. Rev. 2023, 52, 3307. - PubMed
-
- Chemin M., Brun P.‐M., Lecommandoux S., Sandre O., Meins J.‐F. L., Soft Matter 2012, 8, 2867.
-
- Qutbuddin Y., Guinart A., Gavrilović S., Al Nahas K., Feringa B. L., Schwille P., Adv. Mater. 2024, 36, 2311176. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

