Cardiac safety of bedaquiline, delamanid and moxifloxacin co-administered with or without varying doses of sutezolid or delpazolid for the treatment of drug-susceptible TB
- PMID: 40613338
- PMCID: PMC12313457
- DOI: 10.1093/jac/dkaf210
Cardiac safety of bedaquiline, delamanid and moxifloxacin co-administered with or without varying doses of sutezolid or delpazolid for the treatment of drug-susceptible TB
Abstract
Introduction: Many drugs for the treatment of TB prolong the QTc interval, which is associated with an increased risk of developing a life-threatening arrhythmia known as torsades de pointes. Sutezolid and delpazolid are novel oxazolidinones with demonstrated bactericidal activity. We aimed to assess the effects of sutezolid or delpazolid co-administered with bedaquiline, delamanid and moxifloxacin on the QTcF interval (Fridericia's correction). Furthermore, we developed a population pharmacodynamic model to assess the effects of drug exposure on the QTcTBT interval (TB-specific correction).
Methods: Participants were randomized to receive standard-dose bedaquiline, delamanid and moxifloxacin with varying doses of either sutezolid (no sutezolid, 600 mg daily, 1200 mg daily, 600 mg twice daily, 800 mg twice daily) or delpazolid (no delpazolid, 400 mg daily, 800 mg daily, 1200 mg daily, 800 mg twice daily). The QTc interval was determined using weekly ECG assessments.
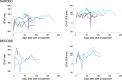
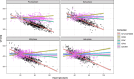
Results: Data from 149 participants, yielding 2373 ECG observations were available for analysis. Nine participants (6.0%) experienced a Grade 3 QTcF prolongation as defined by the Common Terminology Criteria for Adverse Events v5.0. The population pharmacodynamic model predicted a 13.2 ms (95% CI: 10.9-15.3) increase of the QTcTBT in the first week of treatment, but no further increase after that. The exposure of bedaquiline's M2 metabolite was found to drive part of the QTcTBT. No exposure-response relationship was identified for the other drugs investigated.
Conclusions: The drug regimens containing standard doses of bedaquiline, delamanid and moxifloxacin, and varying doses of sutezolid or delpazolid were not found to pose a high cardiac risk in a population without further QTc-relevant risk factors. However, close monitoring of the QTc interval remains essential in patients with TB treated with combination drug therapy with known QTc-prolonging drugs.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
-
- WHO . Implementing the end TB strategy: the essentials, 2022 update. 2022. https://www.who.int/publications/i/item/9789240065093.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

