Effectiveness of switching to doravirine-based antiretroviral therapy: A real-world study in Germany
- PMID: 40613664
- PMCID: PMC12315075
- DOI: 10.1111/hiv.70061
Effectiveness of switching to doravirine-based antiretroviral therapy: A real-world study in Germany
Abstract
Objectives: Doravirine (DOR)-based antiretroviral therapy (ART) has been shown in clinical trials to be effective and well tolerated in treating HIV-1. The EffectiVeness of SwItChing to DORavirine-based Antiretroviral Therapy (VICDOR) study characterized the use, effectiveness and impact on body weight and lipids of DOR-based ART in a virologically suppressed switch population using real-world data from Germany.
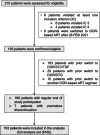
Methods: VICDOR was a multicentre, retrospective chart review study of virologically suppressed adults with HIV in Germany who switched to DOR-based ART between January 2019 and February 2021. Demographic, clinical and laboratory data were collected up to 15 months after the switch.
Results: A total of 193 individuals were included, with a median age of 49 years (range = 21-78), 85.0% were male, 23.8% were obese (BMI ≥30 kg/m2) and 80.8% had at least one comorbidity. Overall, 84.5% switched to DOR/3TC/TDF. The most common reason for switching was to improve tolerability regarding weight gain (37.3%). Of the 161 individuals who remained on DOR and had HIV-1 RNA results, 99.4% remained virologically suppressed after 12 months (with one individual having ≥50 to <200 copies/mL at month 12 and following visit). No virological failures occurred. Median LDL-C change was -7.1 mg/dL (IQR = -23.0 to 8.0; n = 89). Individuals switched to DOR/3TC/TDF had a median weight change of -0.9 kg (IQR = -4.0 to 2.0; n = 50) at month 12; for those switched to DOR/3TC/TDF to improve tolerability regarding weight gain, median weight change was -2.0 kg (IQR = -5.0 to -1.0; n = 23) after 12 months.
Conclusions: DOR-based ART is effective in maintaining virological suppression without evidence of ART-related weight increase in a switch population of individuals with a high prevalence of comorbidities.
Keywords: ART; DOR; DOR/3TC/TDF; HIV; antiretroviral; doravirine; effectiveness.
© 2025 Merck Sharp & Dohme LLC, MVZ München am Goetheplatz, Munich, Praxis Dr. med. Heribert Knechten and The Author(s). HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
C. Wyen potential COIs: invited speaker and advisory board attendee for Gilead, GSK, Janssen, MSD, Pfizer and ViiV Healthcare. M. Sabranski potential COIs: payments or honoraria for presentations, lectures and educational events by MSD, GILEAD, ViiV; travel support and advisory board attendee for Gilead and ViiV; and board member dagnae e.V. C. Jonsson‐Oldebuttel potential COIs: consulting fees from GSK/Viiv Healthcare; payment or honoraria from Abbvie, Gilead, Janssen‐Cilag, GSK/ViiV healthcare; travel or meeting support from Abbvie, Gilead, GSK/ViiV healthcare; advisory or other board attendee for Abbvie, Gilead, GSK/ViiV healthcare, MSD. J. Bogner potential COIs: lecture honoraria‐ AbbVie, AstraZeneca, Pfizer, Boehringer‐Ingelheim, BMS, Gilead, GSK, Janssen, MSD, ViiV, NovoNordisk, Roche; Honoraria for advisory boards‐AbbVie, GSK, MSD, ViiV, Janssen, Pfizer, Gilead. H. Knechten potential COIs: grants, advisory board member and consulting fees from Gilead, MSD, ViiV, Janssen; expert testimony for Gilead, MSD, ViiV; travel/meeting support from Gilead. S. Esser potential COIs: grants, advisory board member, payments/honoraria, travel/meeting support and consulting fees from Gilead, MSD, ViiV, Janssen; leadership/fiduciary role German AIDS Society and Landeskommission AIDS NRW. N. Qurishi potential COIs: consulting fees from Gilead sciences, MSD, ViiV, Janssen‐Cilag; payments/honoraria, advisory board member, meeting/travel support from Gilead sciences, MSD, ViiV. S. Schellberg potential COIs: payments/honoraria from Gilead Sciences, ViiV Healthcare, MSD, AbbVie, Pfizer Inc.; meeting/travel support from Gilead Sciences; advisory board member, Gilead Sciences and ViiV Healthcare. I. Kolobova potential COIs: former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, employed at the time of study, and holds stock for Merck & Co., Inc., Rahway, NJ, USA; current employee and holds stock for GSK/ViiV Healthcare. J.P. Pelz potential COIs: current employee of MSD Sharp & Dohme GmbH, Munich, Germany and holds stock of Merck & Co., Inc., Rahway, NJ, USA. Y.O. Whiteside potential COIs: current employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rayway, NJ,USA and holds stock for Merck & Co., Inc., Rahway, NJ, USA. B. Tadese potential COIs: current employee for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. J.K. Rockstroh potential COIs: consulting fees from Abbvie, Boehringer, Gilead, MSD, ViiV; payments/honoraria from Gilead, Janssen, ViiV; board member for Berlin Cures; EACS Governing Board member; Editor‐in‐Chief of
Figures

References
-
- Curran JW, Jaffe HW, Hardy AM, Morgan WM, Selik RM, Dondero TJ. Epidemiology of HIV infection and AIDS in the United States. Science. 1988;239:610‐616. - PubMed
-
- Berkelman RL, Heyward WL, Stehr‐Green JK, Curran JW. Epidemiology of human immunodeficiency virus infection and acquired immunodeficiency syndrome. Am J Med. 1989;86:761‐770. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services; 2023. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ad...
-
- Deutsche AIDS‐Gesellschaft e.V. (DAIG) . Deutsch‐Österreichische Leitlinien zur antiretroviralen Therapie der HIV‐1‐Infektion, S2k‐Leitlinie. 2021. https://register.awmf.org/de/leitlinien/detail/055-001
-
- Janssen‐Cilag International NV . Summary of product characteristics 25 mg, 100 mg and 200 mg Etravirine.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

