CTCF binding landscape is shaped by the epigenetic state of the N-terminal nucleosome in relation to CTCF motif orientation
- PMID: 40613712
- PMCID: PMC12231596
- DOI: 10.1093/nar/gkaf587
CTCF binding landscape is shaped by the epigenetic state of the N-terminal nucleosome in relation to CTCF motif orientation
Abstract
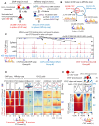
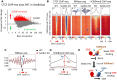
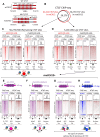
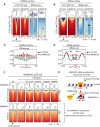
CTCF binding sites serve as anchors for the 3D chromatin architecture in vertebrates. The functionality of these anchors is influenced by the residence time of CTCF on chromatin, which is determined by its binding affinity and its interactions with nucleosomes and other chromatin-associated factors. In this study, we demonstrate that CTCF occupancy is driven by CTCF motifs, strategically positioned at the entry sides of a well-positioned nucleosome, such that, upon binding, the N-terminus of CTCF is oriented towards the nucleosome. We refer to this nucleosome as the CTCF priming nucleosome (CPN). Our analyses suggest that CTCF can more easily displace the CPN if the nucleosome is not marked by CpG methylation or repressive histone modifications. Under these permissive conditions, the N-terminus of CTCF recruits SMARCA5 to reposition the CPN downstream, thereby creating nucleosome-free regions that enhance CTCF occupancy and cohesin stalling. In contrast, when CPNs carry repressive epigenetic marks, CTCF binding is weaker, with no nucleosome displacement or chromatin opening, and cohesin is retained less effectively at CTCF binding sites. We propose that the epigenetic status of CPNs shapes cell-specific CTCF binding patterns, ensuring the maintenance of chromatin architecture throughout the cell cycle.
Published by Oxford University Press on behalf of Nucleic Acids Research 2025.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


Similar articles
-
High-resolution CTCF footprinting reveals impact of chromatin state on cohesin extrusion.Nat Commun. 2025 May 15;16(1):4506. doi: 10.1038/s41467-025-57775-w. Nat Commun. 2025. PMID: 40374602 Free PMC article.
-
Dynamic barriers modulate cohesin positioning and genome folding at fixed occupancy.Genome Res. 2025 Aug 1;35(8):1745-1757. doi: 10.1101/gr.280108.124. Genome Res. 2025. PMID: 40628528 Free PMC article.
-
Binding domain mutations provide insight into CTCF's relationship with chromatin and its contribution to gene regulation.Cell Genom. 2025 Apr 9;5(4):100813. doi: 10.1016/j.xgen.2025.100813. Epub 2025 Mar 20. Cell Genom. 2025. PMID: 40118069 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

