In vivo profiling of the endothelium using 'AngioTag' zebrafish
- PMID: 40613926
- PMCID: PMC12227374
- DOI: 10.1007/s10456-025-09990-8
In vivo profiling of the endothelium using 'AngioTag' zebrafish
Abstract
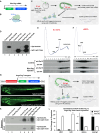
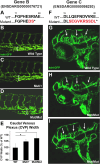
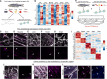
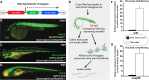
Vascular endothelial cells in vivo are exquisitely regulated by their local environment, which is disrupted or absent when using methods such as FACS sorting of cells isolated from animals or in vitro cell culture. Here, we profile the gene expression patterns of undisturbed endothelial cells in living animals using a novel "AngioTag" zebrafish transgenic line that permits isolation of actively translating mRNAs from endothelial cells in their native environment. This transgenic line uses the endothelial cell-specific kdrl promoter to drive expression of an epitope tagged Rpl10a 60 S ribosomal subunit protein, allowing for Translating Ribosome Affinity Purification (TRAP) of actively translating endothelial cell mRNAs. By performing TRAP-RNAseq on AngioTag animals, we demonstrate strong enrichment of endothelial-specific genes and have uncovered both novel endothelial genes and unique endothelial gene expression signatures for different adult organs. Finally, we generated a versatile "UAS: RiboTag" transgenic line to allow a wider array of different zebrafish cell and tissue types to be examined using TRAP-RNAseq methods. These new tools offer an unparalleled resource to study the molecular identity of cells in their normal in vivo context.
Keywords: Endothelial cell profiling; RiboTag; TRAP-RNAseq; Translatome; Zebrafish.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: Zebrafish husbandry and research protocols were reviewed and approved by the NICHD Animal Care and Use Committee at the National Institutes of Health (ASP # 24 − 015). All animal studies were carried out according to NIH-approved protocols, in compliance with the Guide for the Care and use of Laboratory Animals.
Figures




References
-
- Jung HM, Isogai S, Kamei M, Castranova D, Gore AV, Weinstein BM (2016) Imaging blood vessels and lymphatic vessels in the zebrafish. Methods Cell Biol 133:69–103. 10.1016/bs.mcb.2016.03.023 - PubMed
-
- Swift MR, Weinstein BM (2009) Arterial-venous specification during development. Circ Res 104(5):576–588. 10.1161/CIRCRESAHA.108.188805 - PubMed
-
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM (2001) Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128(19):3675–3683 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

