Resistance and Aerobic Preconditioning Delays Unloading-Induced Multisystemic Physiological Changes: The NEBULA Project
- PMID: 40614062
- PMCID: PMC12227112
- DOI: 10.1096/fj.202501128R
Resistance and Aerobic Preconditioning Delays Unloading-Induced Multisystemic Physiological Changes: The NEBULA Project
Abstract
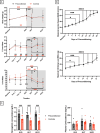
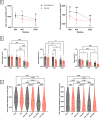
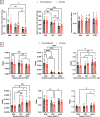
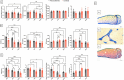
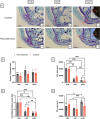
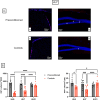
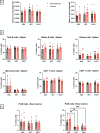
Spaceflight is considered an extreme environment, and from the first days of flight, microgravity causes significant modifications in several physiological systems, particularly the musculoskeletal, cardiovascular, immunological, and nervous systems. To safeguard astronauts' health on upcoming trips, it is crucial to better counteract microgravity's effects using the best prevention methods. With this objective, we launched the NEBULA (Nutrition and Exercise Biology for UnLoaded Astronauts) collaborative research project. The first phase of NEBULA investigates whether a targeted high-intensity pre-flight training program (preconditioning) can delay physiological deconditioning during the initial days of microgravity in a preclinical model. To assess this, sixty 16-week-old male C57BL/6J mice were split into 6 groups (n = 10, each). Half underwent three weeks of preconditioning training (PreC), while the other half remained untrained (Ctrl). Both groups then experienced either 7 or 21 days of hindlimb unloading (HU). Tissue samples-including muscle, bone, tendon, bone marrow, spleen, serum, and brain-were collected at three key time points: post-preconditioning (HU0), after 7 days of HU (HU7), and after 21 days of HU (HU21). At HU0, the PreC group exhibited significantly improved physical performance and enhanced musculoskeletal mass and architecture compared to Ctrl. In the Ctrl group, HU induced severe bone and muscle deconditioning by HU7, which was worsened by HU21. However, in the PreC group, the initial improvements in bone and muscle structure were maintained through HU7 before declining by HU21. Importantly, the high intensity and frequency of training did not negatively impact tendon integrity or immune function and appeared to prevent the decline in adult neurogenesis typically associated with both intense exercise and microgravity. These findings highlight the broad systemic benefits of pre-flight physical conditioning in delaying the adverse effects of reduced mechanical load, such as those experienced during spaceflight or prolonged bed rest. Additionally, they underscore the potential for preconditioning to support more effective countermeasures when physical exercise is unavailable for extended periods.
Keywords: countermeasure; hindlimb unloading; multisystemic; physical exercise; preconditioning; spaceflight.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

