Mitochondrial dysfunction enhances influenza pathogenesis by up-regulating de novo sialic acid biosynthesis
- PMID: 40614187
- PMCID: PMC12227048
- DOI: 10.1126/sciadv.adu3739
Mitochondrial dysfunction enhances influenza pathogenesis by up-regulating de novo sialic acid biosynthesis
Abstract
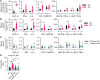
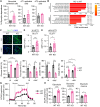
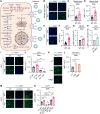
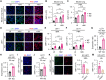
Mitochondrial dysfunction can trigger metabolic adaptations that resemble those induced by influenza A virus (IAV) infection. Here, we show that oxidative phosphorylation (OXPHOS) impairment, modeled by Ndufs4 deficiency, reprograms lung epithelial metabolism to promote IAV pathogenesis. In both Ndufs4 knockout (KO) mice and lung epithelial cells, OXPHOS deficiency increased glycolytic flux, diverting carbons into hexosamine and de novo sialic acid (SIA) biosynthesis pathways. This led to elevated sialylation and enhanced viral attachment. In Ndufs4 KO models, adenosine monophosphate-activated protein kinase signaling was insufficient to blunt this increased metabolic flux. IAV infection further exacerbated this metabolic vulnerability, amplifying SIA and viral burden. Pharmacologic rerouting of glucose carbons with dichloroacetate reduced sialylation, viral replication, and inflammatory responses in Ndufs4 KO models. These findings reveal that mitochondrial dysfunction enhances IAV susceptibility by disrupting energy sensing and fueling viral receptor biosynthesis, highlighting the importance of epithelial metabolism in viral pathogenesis and suggesting metabolic modulation as a potential therapeutic.
Figures


References
-
- Hoffmann R. F., Jonker M. R., Brandenburg S. M., de Bruin H. G., Ten Hacken N. H. T., van Oosterhout A. J. M., Heijink I. H., Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci. Rep. 9, 15047 (2019). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

