Staphylococcus aureus LukMF' targets neutrophils to promote skin and soft tissue infection
- PMID: 40614206
- PMCID: PMC12227067
- DOI: 10.1126/sciadv.adr5240
Staphylococcus aureus LukMF' targets neutrophils to promote skin and soft tissue infection
Abstract
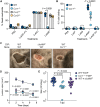
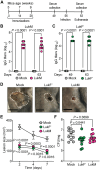
Pathogens have evolved to be highly adapted to their natural host. Community-associated methicillin-resistant Staphylococcus aureus USA300, for instance, is a lineage responsible for the epidemic of skin and soft tissue infections (SSTIs) in humans. Owing to its human tropism, mechanisms that enabled the rise of USA300 as a major skin pathogen remain incompletely defined. By leveraging a rodent-adapted strain of S. aureus, we developed a natural model of SSTIs. We found that LukMF', a pore-forming leukocidin homolog to the human-specific LukSF-PV toxin, drives skin pathology in mice. LukMF' lyses neutrophils via the chemokine receptor CCR1, which in turn fuels inflammatory pathology and microbial survival within the infectious nidus. Ablation of CCR1, depletion of neutrophils, or vaccination with LukMF' all protected mice from skin pathology. Thus, these data support epidemiological studies linking leukocidins with human SSTIs and highlight the power of natural models to unearth potential targets to curtail infections.
Figures



References
-
- Wertheim H. F., Melles D. C., Vos M. C., Van Leeuwen W., Van Belkum A., Verbrugh H. A., Nouwen J. L., The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762 (2005). - PubMed
-
- Ray G. T., Suaya J. A., Baxter R., Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 76, 24–30 (2013). - PubMed
-
- Olaniyi R., Pozzi C., Grimaldi L., Bagnoli F., Staphylococcus aureus-associated skin and soft tissue infections: Anatomical localization, epidemiology, therapy and potential prophylaxis. Curr. Top. Microbiol. Immunol. 409, 199–227 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

