Gaylussacin, a stilbene glycoside, inhibits chronic obstructive pulmonary disease in mice
- PMID: 40614364
- PMCID: PMC12271429
- DOI: 10.1016/j.redox.2025.103744
Gaylussacin, a stilbene glycoside, inhibits chronic obstructive pulmonary disease in mice
Abstract
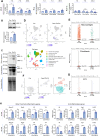
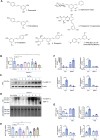
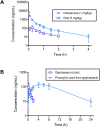
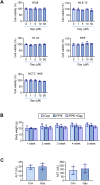
Chronic obstructive pulmonary disease (COPD) is a major cause of human mortality worldwide and is closely associated with chronic inflammation triggered by environmental toxicants such as lead (Pb) and cadmium (Cd). However, the molecular mechanisms linking Pb/Cd exposure to COPD pathogenesis and effective therapeutic strategies remain poorly defined. In this study, we established a mouse model of environmentally induced COPD by exposing mice to Pb/Cd aerosols using a specialized nebulizer system. Pb/Cd exposure led to characteristic COPD-like pathological features, including alveolar damage, mucus hypersecretion, oxidative stress, and apoptosis. Transcriptome analysis of lung tissues revealed upregulation of pro-inflammatory cytokines, chemokines, and lipid metabolism-related genes, with macrophages-particularly those expressing MMP-12-identified as key contributors to pulmonary inflammation. Through a targeted stilbenoid compound screen, we identified gaylussacin as a potent suppressor of Pb/Cd-induced MMP-12 expression in macrophages. Mechanistically, gaylussacin suppressed expression of MMP-12 and inflammatory mediators via activation of SIRT1. In a porcine pancreatic elastase (PPE)-induced emphysema model, oral administration of gaylussacin significantly improved lung function, reduced apoptosis, ROS production, and inflammation. Pharmacokinetic analysis revealed limited oral bioavailability of gaylussacin but efficient conversion to its active metabolite, pinosylvic acid. Toxicological evaluations confirmed negligible toxicity in normal cells derived from various organs and no significant adverse effects in vivo. Collectively, these findings demonstrate that Pb/Cd inhalation promotes COPD pathogenesis through macrophage-driven inflammation mediated by MMP-12 and that gaylussacin mitigates these effects by enhancing SIRT1 activity. This study supports gaylussacin as a promising therapeutic candidate for the treatment of environmentally induced COPD.
Keywords: Chronic obstructive pulmonary disease; Gaylussacin; Heavy metal; Matrix metalloproteinase-12.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- G. B. D. C. o. D. Collaborators Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. May 18 2024;403(10440):2100–2132. doi: 10.1016/S0140-6736(24)00367-2. - DOI - PMC - PubMed
-
- Tachkov K., Kamusheva M., Pencheva V., Mitov K. Evaluation of the economic and social burden of chronic obstructive pulmonary disease (COPD) Biotechnol. Biotechnol. Equip. 2017/07/04 2017;31(4):855–861. doi: 10.1080/13102818.2017.1335616. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

