Structural-functional characterization of the MIRO1-TRAK1 complex
- PMID: 40615373
- PMCID: PMC12227652
- DOI: 10.1038/s41467-025-61174-6
Structural-functional characterization of the MIRO1-TRAK1 complex
Abstract
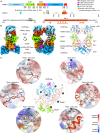
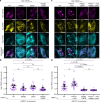
Mitochondrial Rho GTPase (MIRO) features N- and C-terminal GTPase domains (nGTPase and cGTPase) flanking two pairs of EF-hands, and functions as a master scaffold on the outer mitochondrial membrane. It regulates mitochondrial motility by recruiting trafficking kinesin-binding protein (TRAK), which in turn recruits kinesin-1 and dynein-dynactin. The MIRO-TRAK interaction remains incompletely understood. Here, we describe the cryo-electron microscopy structure of TRAK1569-623 bound to MIRO1. The complex forms a dimer, mediated by interactions through the second EF-hand pair, cGTPase, and TRAK1. TRAK1569-623 binds in a cleft between the nGTPase and first EF-hand pair, inserting side chains into hydrophobic pockets of both domains. Another MIRO1-binding site involves TRAK1425-428, which binds in a pocket between the second EF-hand pair and cGTPase. Both binding sites are validated by mutagenesis and binding assays, showing no clear dependence on cofactor conditions (calcium or nucleotide). In cells, both sites contribute to TRAK1's mitochondrial localization.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Fransson, A., Ruusala, A. & Aspenstrom, P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem.278, 6495–6502 (2003). - PubMed
-
- Fransson, S., Ruusala, A. & Aspenstrom, P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun.344, 500–510 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

