Steering artificial photosynthesis via photoinduced conversion of monometallic to bimetallic sites in FeCo nitroprussides
- PMID: 40615385
- PMCID: PMC12227608
- DOI: 10.1038/s41467-025-61129-x
Steering artificial photosynthesis via photoinduced conversion of monometallic to bimetallic sites in FeCo nitroprussides
Abstract
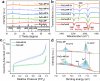
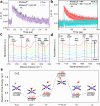
Artificial photosynthesis provides an efficient strategy for solar energy storage via water splitting and CO2 reduction, but it remains a challenge in tuning artificial photosynthesis between these two competing reactions. Herein, we demonstrate photoinduced conversion of monometallic to bimetallic sites in a Fe-Co nitroprusside (FeCo-NP) to steer the reaction path from H2 evolution to CO2 reduction. Monometallic Co sites achieve efficient H2 production with 28.5 mmol g-1 activity and 85.4% selectivity. Photoinduced release of nitrosyl groups from Fe sites generates bimetallic Fe-Co sites, which suppress H2 evolution and enhance CO2 reduction, yielding 31.5 mmol g-1 activity and 87.3% selectivity for C1 products. Mechanistic investigations reveal that monometallic Co sites catalyze H2 evolution via H2O adsorption and O-H cleavage while bimetallic Fe-Co sites facilitate both H2O and CO2 adsorption and subsequent O and C hydrogenation for CO and HCOOH. This work uncovers a strategy to manipulate competing reaction pathways via photoinduced conversion of monometallic to bimetallic sites, which provides unique insights into addressing environmental issues and energy crises.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Rekker, S. et al. Evaluating fossil fuel companies’ alignment with 1.5 °C climate pathways. Nat. Clim. Chang.13, 927–934 (2023).
-
- Lv, J. et al. Solar utilization beyond photosynthesis. Nat. Rev. Chem.7, 91–105 (2023). - PubMed
-
- Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature598, 304–307 (2021). - PubMed
-
- Bonchio, M. et al. Best practices for experiments and reporting in photocatalytic CO2 reduction. Nat. Catal.6, 657–665 (2023).
Grants and funding
- 22471192/National Natural Science Foundation of China (National Science Foundation of China)
- 92161103/National Natural Science Foundation of China (National Science Foundation of China)
- 22022108/National Natural Science Foundation of China (National Science Foundation of China)
- 92461314/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

