A homozygous frameshift variant in the CILK1 gene causes cranioectodermal dysplasia
- PMID: 40615527
- PMCID: PMC12479750
- DOI: 10.1038/s41431-025-01902-0
A homozygous frameshift variant in the CILK1 gene causes cranioectodermal dysplasia
Abstract
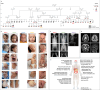
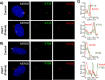
Cranioectodermal dysplasia (CED) is a ciliopathy characterized by skeletal and ectodermal abnormalities, renal failure, and liver fibrosis. Pathogenic variants in genes that encode the intraflagellar transport (IFT) complex components, particularly IFT-A, are responsible for approximately two-thirds of the CED cases. However, the cause of the remaining cases remains unknown. Ciliogenesis-associated kinase 1 (CILK1) is a highly conserved ciliary serine/threonine kinase with an N-terminal catalytic domain responsible for kinase activity and a C-terminal non-catalytic domain that interacts with the IFT-B complex. Biallelic variants in the catalytic domain are associated with lethal skeletal dysplasia, endocrine cerebroosteodysplasia, and short-rib polydactyly syndrome. No human disease has been linked to biallelic variants in the non-catalytic domain. We present a homozygous frameshift variant in the CILK1 gene that affects the distal part of the non-catalytic domain, causing CED in five patients from two pedigrees. All the patients survived into childhood and had disproportionately short stature, skeletal abnormalities, ectodermal dysplasia, renal issues, and liver complications. Functional data from patient-derived cells and the C. elegans model indicate that the variant reduces cilia number, increases cilia length, and disrupts the localization of IFT components. In contrast, the ciliary localization of CILK1 bearing the variant itself remains unaffected. Notably, we rescued the majority of these abnormalities by reintroducing CILK1 into patient-derived cells. Finally, our study describes CILK1 as a novel causal gene and the first non-IFT protein-encoding gene in the etiology of CED, thus expanding the known genotypic, mechanistic, and phenotypic spectrum of CED.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: Subjects were identified through the authors’ clinical practice. This study was approved by the institutional ethics committee on human research at Gazi University, Ankara (approval number#24-27.12.2021). Before participating in the study, all participants or their legal guardians provided informed consent. The methods used in this investigation complied with the ethical guidelines of the relevant committees on human experimentation. The responsible referring physicians at each local site used standard forms to gain permission to include their anonymized medical data, including images, in this cohort.
Figures


References
-
- Tan W, Lin A, Keppler-Noreuil K. Cranioectodermal Dysplasia. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews(®). Seattle, WA: University of Washington, Seattle; 1993. - PubMed
-
- Lai B, Jiang H, Gao Y, Zhou X. Skeletal ciliopathy: pathogenesis and related signaling pathways. Mol Cell Biochem. 2024;479:811–23. - PubMed
-
- Arts HH, Bongers EMHF, Mans DA, van Beersum SEC, Oud MM, Bolat E, et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet. 2011;48:390–5. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

