Homocysteine induces ferroptosis in renal tubular epithelial cells via β-catenin/GPX4 signaling pathway
- PMID: 40615532
- PMCID: PMC12227547
- DOI: 10.1038/s41598-025-09221-6
Homocysteine induces ferroptosis in renal tubular epithelial cells via β-catenin/GPX4 signaling pathway
Abstract
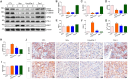
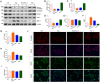
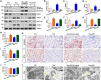
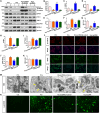
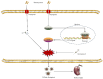
Hyperhomocysteinemia can cause severe damage to kidney. Ferroptosis represents a critical mechanism in the initiation and development of kidney disorders. We focus on the β-catenin/GPX4 signaling pathway to explore how homocysteine influences ferroptosis regulation in renal tubular epithelial cells. C57BL/6J mice were administered drinking water with high level of homocysteine to establish a hyperhomocysteinemia model. In the cell experiments, HKC-8 cells were exposed to homocysteine for a duration of 12 h. Active β-catenin, β-catenin, GPX4, FTH1, and KIM-1 were detected using Western blotting; Biochemical assays were conducted to measure lipid ROS, Fe2+, and GSH; GPX4 and β-catenin were detected through immunohistochemistry and immunofluorescence techniques; Mitochondrial damage was examined using transmission electron microscopy; ChIP analysis, coupled with dual-luciferase reporter gene assays, was employed to investigate the relationship between β-catenin protein and GPX4 gene promoter. Our findings revealed that homocysteine disrupted β-catenin signaling, inhibited GPX4 expression in renal tubular epithelial cells, subsequently promoted ferroptosis. Overexpression of β-catenin or GPX4 inhibited ferroptosis induced by homocysteine, and β-catenin regulated GPX4 expression in renal tubular epithelial cells. Further assays demonstrated that GPX4 acted as a target gene of β-catenin. In conclusion, homocysteine elicits ferroptosis in renal tubular epithelial cells by disrupting β-catenin signaling and inhibiting its target gene, GPX4.
Keywords: Ferroptosis; GPX4; Hyperhomocysteinemia; Renal tubular epithelial cells; Β-catenin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This study protocol was reviewed and approved by the Animal Care and Use Committee of University of South China (Ethics approval number: USC2023XS087).
Figures



Similar articles
-
Homocysteine induces ferroptosis in cardiomyocytes by disrupting β-catenin/GPX4 pathway.PLoS One. 2025 Aug 6;20(8):e0329792. doi: 10.1371/journal.pone.0329792. eCollection 2025. PLoS One. 2025. PMID: 40768425 Free PMC article.
-
Homocysteine promotes cardiomyocyte hypertrophy through inhibiting β-catenin/ FUNDC1 mediated mitophagy.Sci Rep. 2025 Jul 1;15(1):22207. doi: 10.1038/s41598-025-06772-6. Sci Rep. 2025. PMID: 40593088 Free PMC article.
-
Protein phosphatase 2A-B55β mediated mitochondrial p-GPX4 dephosphorylation promoted sorafenib-induced ferroptosis in hepatocellular carcinoma via regulating p53 retrograde signaling.Theranostics. 2023 Jul 31;13(12):4288-4302. doi: 10.7150/thno.82132. eCollection 2023. Theranostics. 2023. PMID: 37554285 Free PMC article.
-
Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer.Int J Mol Sci. 2025 Jul 4;26(13):6452. doi: 10.3390/ijms26136452. Int J Mol Sci. 2025. PMID: 40650229 Free PMC article. Review.
-
Targeting Ferroptosis in Tumors: Novel Marine-Derived Compounds as Regulators of Lipid Peroxidation and GPX4 Signaling.Mar Drugs. 2025 Jun 19;23(6):258. doi: 10.3390/md23060258. Mar Drugs. 2025. PMID: 40559667 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2021JJ40476/Natural Science Foundation of Hunan Province
- 2022JJ30528/Natural Science Foundation of Hunan Province
- 2021JJ40506/Natural Science Foundation of Hunan Province
- 21B0414/Scientific Research Project of Hunan Provincial Department of Education
- C202303019182/Scientific Research Fund Project of Hunan Provincial Health Commission
LinkOut - more resources
Full Text Sources
Miscellaneous

