Human thalamocortical structural connectivity develops in line with a hierarchical axis of cortical plasticity
- PMID: 40615590
- PMCID: PMC12321582
- DOI: 10.1038/s41593-025-01991-6
Human thalamocortical structural connectivity develops in line with a hierarchical axis of cortical plasticity
Abstract
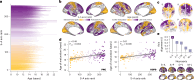
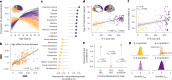
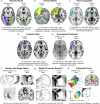
Human cortical development follows a hierarchical, sensorimotor-to-association sequence. The brain's capacity to enact this sequence indicates that it relies on unknown mechanisms to regulate regional differences in the timing of cortical maturation. Given evidence from animal systems that thalamic axons mechanistically regulate periods of cortical plasticity, here we evaluate in humans whether the development of structural connections between the thalamus and cortex aligns with cortical maturational heterochronicity. By deriving a new tractography atlas of human thalamic connections and applying it to diffusion data from three youth samples (8-23 years; total n = 2,676), we demonstrate that thalamocortical connectivity matures in a generalizable manner along the cortex's sensorimotor-association axis. Associative cortical regions with thalamic connections that take the longest to mature exhibit neurochemical, structural and functional signatures of protracted developmental plasticity as well as heightened sensitivity to the socioeconomic environment. This work highlights the role of the thalamus in the expression of hierarchical periods of cortical developmental plasticity and environmental receptivity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Grants and funding
- R01MH133334/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH120482/MH/NIMH NIH HHS/United States
- U24 NS130411/NS/NINDS NIH HHS/United States
- U24NS130411/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 MH132934/MH/NIMH NIH HHS/United States
- R01 MH124045/MH/NIMH NIH HHS/United States
- T32 DC000038/DC/NIDCD NIH HHS/United States
- R01 MH131638/MH/NIMH NIH HHS/United States
- R01MH120482/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- T32 MH019112/MH/NIMH NIH HHS/United States
- R01 NS112274/NS/NINDS NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- R01MH133843/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R00MH127293/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH123563/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R37 MH125829/MH/NIMH NIH HHS/United States
- F31 HD111139/HD/NICHD NIH HHS/United States
- R01 MH123550/MH/NIMH NIH HHS/United States
- R01 MH123563/MH/NIMH NIH HHS/United States
- DGE-1845298/National Science Foundation (NSF)
- T32 MH016804/MH/NIMH NIH HHS/United States
- R00 MH127293/MH/NIMH NIH HHS/United States
- R01EB022572/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH123550/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH133843/MH/NIMH NIH HHS/United States
- R01 MH113550/MH/NIMH NIH HHS/United States
- R01MH120174/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH119219/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH113550/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- T32MH016804/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH130578/MH/NIMH NIH HHS/United States
- R01MH131638/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- S10OD023495/U.S. Department of Health & Human Services | NIH | NIH Office of the Director (OD)
- R01MH112847/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01NS112274/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 MH120174/MH/NIMH NIH HHS/United States
- R01MH119185/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- S10 OD023495/OD/NIH HHS/United States
- R01 MH133334/MH/NIMH NIH HHS/United States
- P50 MH109429/MH/NIMH NIH HHS/United States
- T32MH019112/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH119219/MH/NIMH NIH HHS/United States
- R37MH125829/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH132934/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH130578/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH119185/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

