Quantifying Experimental Variability in Shear-Induced Hemolysis to Support Uncertainty-Aware Hemolysis Models
- PMID: 40615756
- PMCID: PMC12457503
- DOI: 10.1007/s10439-025-03786-z
Quantifying Experimental Variability in Shear-Induced Hemolysis to Support Uncertainty-Aware Hemolysis Models
Abstract
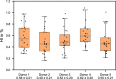
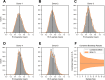
Purpose: Numerical hemolysis models rely on experimental data to fit parameters and predict hemolysis under various conditions. However, existing experiments often use few replicates per condition, leaving inherent variability largely unaddressed. This can lead to oversimplified models that fail to capture the true nature of hemolysis. Here, we quantify intra- and inter-donor variability at a single, well-defined shear stress and exposure time and examine how sample size affects measurement precision METHODS: Human blood from five healthy donors was subjected to a fixed shear stress and exposure time condition. For each donor, 20 independent measurements were performed to calculate a hemolysis index (HI). Intra-donor variability (variation within a single donor's measurements) and inter-donor variability (variation between donor means) were compared. Additionally, bootstrap analyses were used to explore the effect of the sample size on the confidence intervals of the mean HI.
Results: Intra-donor variability was approximately four times higher than inter-donor variability, indicating that most of the uncertainty originated from within a single donor's set of samples rather than between donors. Increasing the sample size from 2 to 20 replicates substantially narrowed the confidence intervals of the mean hemolysis estimate, suggesting that commonly used small sample sizes may underrepresent the true variability in hemolysis measurements.
Conclusion: Intra-donor variability is a significant driver of uncertainty in hemolysis measurements at a fixed shear stress and exposure time condition, surpassing differences among donors. Obtaining robust and reliable hemolysis estimates requires increasing the number of replicate measurements to reduce uncertainty. Integrating these insights into future experimental designs and uncertainty-aware hemolysis models will improve the reliability of in silico predictions and inform safer, more effective blood-contacting medical device designs.
Keywords: Experimental hemolysis; Inter-variability; Intra-variability; Uncertainty quantification.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no competing interests to declare that are relevant to the content of this article.
Figures


References
-
- Shapira, Y., M. Vaturi, and A. Sagie. Hemolysis associated with prosthetic heart valves: a review. Cardiol Rev. 17:121–124, 2009. 10.1097/CRD.0b013e31819f1a83. - PubMed
-
- Shah, P., U. S. Tantry, K. P. Bliden, and P. A. Gurbel. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant. 36:1164–1173, 2017. 10.1016/j.healun.2017.05.008. - PubMed
-
- Viceconti, M., L. Emili, P. Afshari, E. Courcelles, C. Curreli, N. Famaey, et al. Possible Contexts of Use for In Silico Trials Methodologies: A Consensus-Based Review. IEEE J Biomed Health Inform. 25:3977–3982, 2021. 10.1109/JBHI.2021.3090469. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

