Low-dose interleukin-2 in patients with mild to moderate Alzheimer's disease: a randomized clinical trial
- PMID: 40615880
- PMCID: PMC12231701
- DOI: 10.1186/s13195-025-01791-x
Low-dose interleukin-2 in patients with mild to moderate Alzheimer's disease: a randomized clinical trial
Abstract
Background: We previously documented that regulatory T cells (Tregs) immunomodulatory mechanisms are compromised in Alzheimer's disease (AD), shifting the immune system toward a pro-inflammatory response. However, Tregs are a potentially restorable therapeutic target in AD. In this study, we evaluated the safety and efficacy of two dosing frequencies of low-dose Interleukin-2 (IL-2) in expanding Tregs to modify disease progression in AD individuals.
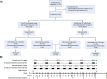
Methods: In this phase 2a, randomized, double-blind, placebo-controlled study, 38 participants were assigned to receive subcutaneous IL-2 (10^6 IU/day) for five days, administered either every 4 weeks (IL-2 q4wks) or every 2 weeks (IL-2 q2wks), versus placebo, for 21 weeks, followed by 9 weeks of observation. The primary endpoints were the incidence and severity of adverse events. For the secondary endpoints, changes in Treg numbers and suppressive functions were evaluated. Exploratory endpoints included changes in plasma inflammatory mediators, CSF AD-related biomarkers, and clinical scales.
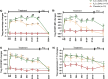
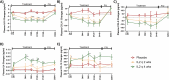
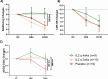
Results: Of the 38 participants, 9 received IL-2 q4wks, 10 received IL-2 q2wks, and 19 received placebo. All participants completed the trial with no serious adverse events or deaths. Both IL-2 dosing regimens increased Treg numbers and suppressive function, but IL-2 q4wks treatment exhibited superiority in enhancing Treg percentage and Foxp3 mean fluorescent intensity. In longitudinal analysis of 45 inflammatory mediators, IL-2 q4wks administration demonstrated greater efficacy in alleviating the plasma inflammatory mediators CCL2, CCL11, and IL-15, while enhancing IL-4 and CCL13 levels. A significant improvement in CSF Aβ42 levels (p = 0.045 vs. placebo) on Day 148 was observed following IL-2 q4wks administration, compared to placebo. While CSF NfL increased by 217 pg/ml in placebo recipients, it remained stable in the IL-2 q4wks group (p = 0.060, IL-2 q4wks vs. placebo). The adjusted mean change from baseline in the ADAS-cog score at week 22 indicated a trend toward slower clinical progression in IL-2 q4wks recipients compared to placebo (p = 0.061).
Conclusions: The IL-2 immunotherapeutic strategy was safe and well-tolerated. IL-2 q4wks effectively expanded Treg populations, leading to modification in inflammatory mediators and CSF Aβ42 levels, while also showing promising trends on clinical scales. These findings provide a foundation for further investigation of low-dose IL-2 as a potential treatment for Alzheimer's Disease.
Trial registration: ClinicalTrials.gov Identifier: NCT06096090, Registration Date: 10-17-2023.
Keywords: Alzheimer’s Disease; Clinical trial; Immune system; Immunotherapy; Inflammation; Treg.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The trial was approved by the Houston Methodist Research Institute (HMRI) institutional review board (IRB) committee. All patients signed informed consent prior to study enrolment. Consent for publication: Not applicable. Competing interests: AF, ADT, JCM, JRT declares a conflict of interest as a consultant with Coya Therapeutics, Inc. SHA declares a conflict of interest as a consultant with Implicit Bioscience and scientific advisory board chair of Coya Therapeutics, Inc. DRB declares a conflict of interest as a consultant with Implicit Bioscience and Coya Therapeutics, Inc. MG is an employee of Global R&D Partners, LLC, which received consulting fees from HMRI. CD is an employee of CSD Biostatistics, Inc, which received consulting fees from HMRI. Remaining authors have no conflict of interest.
Figures

References
-
- Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature. 2017;552(7685):355–61. - PubMed
-
- Liu Y, Dai Y, Li Q, Chen C, Chen H, Song Y, et al. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci Lett. 2020;736:135279. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

