Proteomic discovery analysis of quantitatively assessed emphysema in the general population. The MESA Lung Study
- PMID: 40616090
- PMCID: PMC12228189
- DOI: 10.1186/s12931-025-03312-8
Proteomic discovery analysis of quantitatively assessed emphysema in the general population. The MESA Lung Study
Abstract
Background: Pulmonary emphysema occurs frequently in older adults, often without airflow limitation. Its presence predicts symptoms, respiratory hospitalizations and deaths, and all-cause mortality. Proteomics may provide further insights into emphysema pathogenesis and inform therapeutic targets.
Objective: We performed a proteomic discovery analysis of percent emphysema on computed tomography (CT) in a population-based, multiethnic sample from the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Replication was performed in two chronic obstructive pulmonary disease (COPD)-based studies, the SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) and the Genetic Epidemiology of COPD (COPDGene) Study.
Methods: MESA recruited participants from the general population in 2000-02. The MESA Lung Study performed full-lung CT scans in 2010-12. Percent emphysema was defined as the percentage of lung voxels < -950 Hounsfield units. Over 7,200 plasma aptamers were measured via SomaScan. Cross-sectional linear and least absolute shrinkage and selection operator (LASSO) regression models were adjusted for demographics, anthropometrics, smoking, renal function, and scanner parameters. Statistical significance was defined as a false discovery rate p-value < 0.05. Gene Ontology (GO)/Reactome enrichment analyses were performed. LASSO-selected proteins' predictive performance was evaluated.
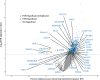
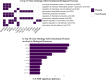
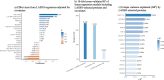
Results: Among 2,504 participants in the MESA Lung Study, mean age was 69.4 years, 1,291 had ever smoked, and median percent emphysema-like lung was 1.4%. In total, 1,234 aptamers were significantly associated with percent emphysema in the MESA Lung Study, and 35 replicated in the SPIROMICS and COPDGene Studies. Novel associations included protein family with sequence similarity (FAM) 177A1, syntenin-2, ubiquitin carboxyl-terminal hydrolase 25, and uncharacterized protein C20orf173. Previously identified emphysema-associated proteins included soluble advanced glycosylation end product-specific receptor (sRAGE), protein S100-A12, high mobility group protein B1, and roundabout homolog 2. Enrichment analyses identified 40 GO biological processes, including chemokine production and regulation and cell-cell adhesion and regulation, and two Reactome pathways, including RAGE signaling. In tenfold cross-validation, novel proteins were largely retained by LASSO (R2 = 5.4%), improved overall model performance (R2 = 24.8%), and uniquely explained greater variance in percent emphysema.
Conclusions: This analysis in a general population sample identified novel and previously characterized proteins whose functional roles were validated by GO/Reactome enriched pathways, offering new insights into emphysema pathophysiology and therapeutics.
Keywords: Biomarkers; Emphysema; Proteomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: RD, RFD, CJG, LR have nothing to declare. All support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.): CBC declares grants to UCLA from the NIH/NHLBI, Foundation NIH, and COPD Foundation, NBA, AGB, AWM declare grants from NIH/NHLBI. CKG declares PR202907 W81XWH2110216. ATH declares subcontract funding from the NHLBI from UCSF to the University as a clinical site for the SPIROMICS program, to enroll and characterize subjects in the study, and from FNIH for funding to the University as a clinical site for the SPIROMICS program to enroll and characterize subjects in the study. EAH declares payment to the University of Iowa from the NIH. RP declares grants from NHLBI and COPD Foundation. JSP declares payment to institution from NIH/NHLBI. WSP declares payment to Johns Hopkins University from NIH. KAP declares Awards Number U01 HL089897, Number U01 HL089856, R01 HL137995 and R01 HL129937. EKS declares institutional grant support from NIH. YS declares NIH/NHLBI grant 5R01HL077612. PGW declares NIH and COPD Foundation support for SPIROMICS. JLC declares payment to institution from NIH/NHLBI and COPDGene Foundation. RGB declares grants to institution from NIH/NHLBI, COPDGene Foundation, and Foundation for the NIH. DEG declares T32 grant to institution from NIH/NHLBI. Grants or contracts from any entity not indicated above: IZB declares investigator-initiated grants from Theravance and Viatris, Aerogen, Alpha-1 Foundation, Johnny Carson’s Foundation, Takeda, and Amgen. ERB declares clinical trials and contracts administered through his employer, the Mayo Clinic and the University of Arizona through July 2023 for Astra Zeneca, Sanofi Genzyme, and TEVA. CKG declares several grants to her institution including R01 HL093096, Pulmonary Fibrosis Foundation Award, and 5UL1 TR001873 Boehringer Ingelheim (PI renamed in 2023). JSK declares grants from NHLBI, Chest. RP declares a grant from Dept of Veterans Affairs Merit. WSP, JIR declare grants from NIH. EKS declares institutional grant support from Bayer and Northpond Laboratories. JMW declares R01HL148215; UH3HL152323; R01HL153460; R01HL162705; R35HL166433 from NIH/NHLBI, 1I01BX005957 from Department of Veteran Affairs, and contracted research support from ARCUS-Med, Mereo BioPharma, Medscape, Verona Pharma, Grifols, Alpha-1 Foundation, InhibrX, and American Lung Association. JLC declares payment to institution from NIH/NHLBI, Department of Veterans Affairs, and Department of Defense. KEW declares NIH MESA contract. RGB declares grant to institution from American Lung Association. Royalties or licenses: CBC declares Cambridge University Press book author personal fees. Consulting fees: CBC declares consulting fees for AstraZeneca Scientific Advisor – ATHLOS study. IZB declares consulting fees for the advisory boards of Astra Zeneca, Sanofi/Regeneron, Verona Pharma, Inhibrx, Takeda, Genentech, Aerogen, Therevance and Viatris, and speaker bureau fees for Grifols. ERB declares consulting fees for Astra Zeneca less than $2500. CKG declares consulting fees for Rejuveron Telomere Therapeutics AG (last received 2023). RP declares consulting fees for Partner Therapeutics. PGW declares consulting fees for Sanofi, Roche, AnaptysBio, and Abbvie. JLC declares consulting fees paid to institution from AstraZeneca. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: CBC declares GlaxoSmithKline and Chulalongkorn University, Bangkok Thailand personal fees. IZB declares payment or honoraria for the advisory boards of Astra Zeneca, Sanofi/Regeneron, Verona Pharma, Inhibrx, Takeda, Genentech, Aerogen, Therevance and Viatris, and speaker bureau fees for Grifols. CKG declares payment or honoraria for Interstitial Lung Disease CME; Stanford University (2024). AWM declares payment for editorial services from the American Journal of Respiratory Critical Care Medicine VEO declares compensation for meeting attendance and presentation at the Respiratory Young Investigator’s Forum paid by National Jewish Health. KEW declares payment or honoraria from Borhringer Ingelheim. Payment for expert testimony: CBC declares medicolegal personal fees from various law firms. Support for attending meetings and/or travel: CBC declares support for AstraZeneca ATHLOS EU Investigator Meeting, AstraZeneca ATHLOS US Investigator Meeting, and AstraZeneca ATHLOS APAC Investigator Meeting. EAH declares support to the University of Iowa for travel to ATS from the NIH. DEG declares support to Columbia University for travel to ATS from the NIH. RPB declares support for travel $250 to a meeting from SomaLogic. Patents planned, issued or pending: CBC declares an issued patent Systems and methods for determining time constant for oxygen uptake kinetics. United States Provisional Patent Application. 63/409,865. September 26, 2022. CKG declares a pending patient US application no 63/565,938 filed 3/15/24 for qPCR assay for measuring telomere length filed by Columbia University. JMW declares PCT/GB2021/050658 with Mereo BioPharma. DEG declares issued patents Uniformly pressing micro-valve system for controlling path of fluids in miniaturized capillary connections and methods of fabrication. U.S. Patent Publication Number 11931741-B2. 2024, Mar., Combined micro valve and pressing member for controlling path of fluids in miniaturized capillary connections. U.S. Design Patent Number D970041-S. 2022, May., Integrated modular unit containing one or more analyte concentrator-microreactor devices to be coupled to a cartridge-cassette and methods of operation. U.S. Patent Publication Number 11198118-B2. 2021, Dec., Analyte concentrator-microreactor device. U.S. Design Patent Number D923817-S. 2021, Jun., Micro valve for controlling path of fluids in miniaturized capillary connections. U.S. Design Patent Number D919833-S. 2021, May., and Analyte concentrator-microreactor device. U.S. Design Patent Number D857231-S. 2019, Aug. Participation on a Data Safety Monitoring Board or Advisory Board: CBC declares personal fees for NUVAIRA Chair of Clinical Events Committee, Horizon Therapeutics Event Adjudication Committee, MGC Diagnostics Scientific Advisory Board, Chiesi Scientific Advisory Board, Herbalife Nutrition Institute Editorial Board Member, Respiree Scientific Advisory Board Member, Aer Therapeutics Scientific Advisory Board Member, Genentec Chair of Clinical Events Committee, RS Biotherapeutics Therapeutic Expert Council, and Verona Scientific Advisory Board. PG declares serving for the Medical Advisory Board of SomaLogic, for which he does not accept any remuneration. VEO declares participation on Independent Data Monitoring Board for Regeneron and Sanofi. JMW declares personal fees for advisory work from AstraZeneca, Takeda, GSK, Bavarian Nordic, Krystal Biotech, Sanofi, and Verona Pharma. JLC declares participation with Novartis paid to institution and Genentech paid to him. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: CKG declares unpaid roles as NIH ClinGen Pulmonary Executive Co-Chair and NIH ClinGen ILD GCEP Co-Chair. EAH declares unpaid member of Photon Counting CT advisory committee for Siemens Healthineers. JLC declares American Thoracic Society role paid to him. RGB declares unpaid role in COPD Foundation. Stock or stock options: CKG declares stock vesting in Rejuvenation Technologies Inc. until 08/2026. EAH declares he is a founder and shareholder of VIDA Diagnostics. JMW declares stock options in Alveolus Bio. Receipt of equipment, materials, drugs, medical writing, gifts or other services: JMW declares support for medical writing for Takeda, GSK, and Verona Pharma. Other financial or non-financial interests: ERB declares all grants, contracts, and payments administered by his employer, the Mayo Clinic, from the NIH, NHLBI, SPIROMICS, SARP, EPIPHANY R01, and PrecISE.
Figures
References
-
- World Health Organization. The top 10 causes of death, 2019. Geneva, Switzerland: WHO; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 2024 June.
-
- Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28(5):555–62. - PubMed
-
- Auerbach O, Hammond EC, Garfinkel L, Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972;286(16):853–7. - PubMed
-
- Sanders C, Nath PH, Bailey WC. Detection of emphysema with computed tomography. Correlation with pulmonary function tests and chest radiography. Invest Radiol. 1988;23(4):262–6. - PubMed
MeSH terms
Grants and funding
- R01HL159081/HL/NHLBI NIH HHS/United States
- T32 HL144442/HL/NHLBI NIH HHS/United States
- R01 HL137995/HL/NHLBI NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- T32-HL144442/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 HL159081/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- 75N92023D00011/NH/NIH HHS/United States
- 1R01HL137995/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- HHSN268200900013C-HHSN268200900020C/National Heart, Lung, and Blood Institute,United States
- 75N92023D00011/HL/NHLBI NIH HHS/United States
- R01-HL077612/HL/NHLBI NIH HHS/United States
- R01-HL130506/HL/NHLBI NIH HHS/United States
- UL1-TR-000040/TR/NCATS NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- R01 HL130506/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous