The Enzyme Glucose-1-Phosphate Thymidylyltransferase RmlA Plays a Crucial Role in the Pathogenesis of Pectobacterium actinidiae GX1
- PMID: 40616237
- PMCID: PMC12227328
- DOI: 10.1111/mpp.70118
The Enzyme Glucose-1-Phosphate Thymidylyltransferase RmlA Plays a Crucial Role in the Pathogenesis of Pectobacterium actinidiae GX1
Abstract
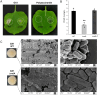
Pectobacterium actinidiae is one of the primary pathogens that causes summer canker disease in kiwifruit, yet its pathogenic mechanisms remain unknown. The exopolysaccharide PCAP-1a, isolated from the fermentation broth of P. actinidiae strain GX1, exhibits notable cytotoxicity and acts as a virulence factor facilitating host infection. Genome-wide analysis revealed a 21-gene cluster responsible for the biosynthesis of exopolysaccharides in GX1. Homologous recombination was used to systematically knock out these genes, which led to the identification of RmlA as a key protein in the synthesis of the PCAP-1a precursor. The deletion of the rmlA gene significantly affected the yield of PCAP-1a and resulted in a direct reduction in GX1 pathogenicity. Further studies revealed that mutations in the substrate binding site of RmlA weakened its capacity to bind G-1-P and dTTP, which led to markedly reduced pathogenicity in the corresponding complemented strains. This study indicates that the exopolysaccharide PCAP-1a serves as a virulence factor in the pathogenesis of GX1, and its biosynthesis depends on the polysaccharide synthesis gene rmlA and the substrate binding activity of its encoded protein.
Keywords: Pectobacterium actinidiae; biosynthesis‐related gene; genome; pathogenicity; polysaccharide.
© 2025 The Author(s). Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
RetS-mediated environmental sensing coordinates TetR-dependent regulation of type III secretion system and virulence in Pseudomonas syringae pv. actinidiae.Appl Environ Microbiol. 2025 Jul 23;91(7):e0049425. doi: 10.1128/aem.00494-25. Epub 2025 Jun 10. Appl Environ Microbiol. 2025. PMID: 40492733 Free PMC article.
-
Genomic Variation and Host Interaction among Pseudomonas syringae pv. actinidiae Strains in Actinidia chinensis 'Hongyang'.Int J Mol Sci. 2022 Aug 28;23(17):9743. doi: 10.3390/ijms23179743. Int J Mol Sci. 2022. PMID: 36077140 Free PMC article.
-
Novel insights into the genetic basis and transcriptomic elucidation of virulence attenuation in Pseudomonas syringae pv. actinidiae variants.Virulence. 2025 Dec;16(1):2543983. doi: 10.1080/21505594.2025.2543983. Epub 2025 Aug 22. Virulence. 2025. PMID: 40844836 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Arayes, M. A. , Mabrouk M. E. M., Sabry S. A., et al. 2023. “Exopolysaccharide Production From Alkalibacillus sp. w3: Statistical Optimization and Biological Activity.” Biologia 78, no. 1: 229–240.
-
- Aslam, S. N. , Newman M. A., Erbs G., et al. 2008. “Bacterial Polysaccharides Suppress Induced Innate Immunity by Calcium Chelation.” Current Biology 18, no. 14: 1078–1083. - PubMed
-
- Azimi, A. , Aslanimehr M., Yaseri M., Shadkam M., and Douraghi M.. 2020. “Distribution of smf‐1, rmlA, spgM and rpfF Genes Among Stenotrophomonas maltophilia Isolates in Relation to Biofilm‐Forming Capacity.” Journal of Global Antimicrobial Resistance 23: 321–326. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

