NOD1 promotes leukocyte clearance and limits inflammation in female mice during obesity-associated acute lung injury
- PMID: 40616268
- PMCID: PMC12227658
- DOI: 10.14814/phy2.70446
NOD1 promotes leukocyte clearance and limits inflammation in female mice during obesity-associated acute lung injury
Abstract
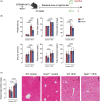
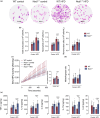
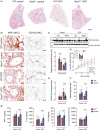
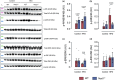
Obesity is associated with metabolic inflammation, which includes changes to innate immune responses relevant to acute lung injury. NOD1 is a cytosolic pattern recognition receptor involved in sensing bacterial peptidoglycan and has been linked to metabolic inflammation. However, its role in obesity-associated acute lung injury, especially in females, remains unclear. Here, we investigated the impact of NOD1 deficiency on pulmonary inflammation in female mice subjected to a high-fat diet and lipopolysaccharide-induced acute lung injury. Compared to wild-type controls, obese Nod1-/- mice showed reduced leukocyte and neutrophil numbers in the bronchoalveolar lavage (BAL), but increased BAL levels of TNF-α, IL-1β, IL-6, IL-17A, and IL-22, suggesting impaired neutrophil clearance. In the lung tissue, NOD1 deficiency during obesity led to elevated neutrophil accumulation, increased myeloperoxidase activity, reduced CD163+ macrophages, and enhanced β-galactosidase activity. Gene expression analysis revealed upregulation of chemokines, adhesion molecules, and inflammasome components, alongside downregulation of M2 polarization markers. Additionally, obese Nod1-/- mice showed higher NF-κB and ERK1/2 activation and lower p38 phosphorylation. These findings indicate that NOD1 regulates leukocyte dynamics, inflammation, and macrophage function in the obese lung. We identify NOD1 as a key protective modulator of pulmonary immune responses during acute lung injury under metabolic stress.
Keywords: NOD1; acute lung injury; inflammation; obesity.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Hypothermia protects against ventilator-induced lung injury by limiting IL-1β release and NETs formation.Elife. 2025 Jun 24;14:RP101990. doi: 10.7554/eLife.101990. Elife. 2025. PMID: 40553503 Free PMC article.
-
Retinoic acid differently modulates NOD1/NOD2-mediated inflammatory responses in human macrophage subsets.Front Immunol. 2025 Jul 1;16:1609763. doi: 10.3389/fimmu.2025.1609763. eCollection 2025. Front Immunol. 2025. PMID: 40666513 Free PMC article.
-
IL-17A drives a fibroblast-neutrophil-NET axis to exacerbate immunopathology in the lung with diffuse alveolar damage.Front Immunol. 2025 Jun 11;16:1574246. doi: 10.3389/fimmu.2025.1574246. eCollection 2025. Front Immunol. 2025. PMID: 40568586 Free PMC article.
-
Leptin and Acute Lung Disorders.Compr Physiol. 2025 Aug;15(4):e70025. doi: 10.1002/cph4.70025. Compr Physiol. 2025. PMID: 40635334 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Ait Yahia, S. , Audousset, C. , Alvarez‐Simon, D. , Vorng, H. , Togbe, D. , Marquillies, P. , Delacre, M. , Rose, S. , Bouscayrol, H. , Rifflet, A. , Quesniaux, V. , Boneca, I. G. , Chamaillard, M. , & Tsicopoulos, A. (2021). NOD1 sensing of house dust mite–derived microbiota promotes allergic experimental asthma. Journal of Allergy and Clinical Immunology, 148(2), 394–406. - PubMed
-
- Aoshiba, K. , Tsuji, T. , Kameyama, S. , Itoh, M. , Semba, S. , Yamaguchi, K. , & Nakamura, H. (2013). Senescence‐associated secretory phenotype in a mouse model of bleomycin‐induced lung injury. Experimental and Toxicologic Pathology, 65(7), 1053–1062. - PubMed
-
- Cho, W. H. , Oh, J. Y. , Yeo, H. J. , Han, J. , Kim, J. , Hong, S. B. , Chung, C. R. , Park, S. H. , Park, S. Y. , Sim, Y. S. , Cho, Y. J. , Park, S. , & Kang, B. J. (2018). Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: Analysis of the national registry. Journal of Critical Care, 48, 453–457. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

