De-Escalation Treatment Strategies From Natalizumab in Patients With Relapsing Multiple Sclerosis in Austria
- PMID: 40616293
- PMCID: PMC12227798
- DOI: 10.1111/ene.70282
De-Escalation Treatment Strategies From Natalizumab in Patients With Relapsing Multiple Sclerosis in Austria
Abstract
Objectives: This study aims to assess the efficacy of de-escalating from natalizumab (NTZ) to cladribine (CLAD), dimethyl fumarate (DMF), fingolimod (FTY), ponesimod (PONE), siponimod (SIPO) and teriflunomide (TERI).
Material and methods: We analyzed data from 388 patients in the Austrian MS Treatment Registry who initiated NTZ treatment and remained on therapy for at least 3 months before switching to one of the moderately effective therapies within 1 year. Patients were required to remain on the de-escalation therapy for at least 3 months.
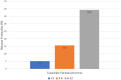
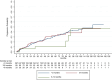
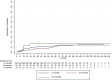
Results: Over a mean treatment duration of 42 months, the estimated ARR (annualized relapse rate) was 0.22 for highly effective therapy and 0.36 for de-escalation therapies over 61 months (p = 0.009). EDSS scores increased significantly from 2.8 to 3.1 during de-escalation (p < 0.001). Relapse probability during the treatment gap varied by interval: 14 patients (5.2%) in the < 3 months group, 14 patients (15.7%) in the 3-6 months group, and 13 patients (39.4%) in the 6-12 months group (p < 0.001). Male sex, lower baseline ARR (prior to the initiation of hDMT) and during transition, older age, shorter disease duration, and lower EDSS scores at both baseline and post-transition were significantly associated with a reduced risk of relapse and longer time to first relapse following de-escalation.
Conclusions: Our findings reveal an increased risk of relapses and EDSS worsening following de-escalation from NTZ. Additionally, relapse probability and EDSS progression were influenced by ARR during transition and EDSS scores at the end of the transition period.
Keywords: de‐escalation; efficacy; multiple sclerosis; natalizumab; real‐world; registry.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Michael Guger received support and honoraria for research, consultation, lectures, and education from Alexion, Almirall, Bayer, Biogen, Bristol‐Myers‐Squibb, Celgene, Genzyme, Horizon, Janssen‐Cilag, MedDay, Merck, Neuraxpharm, Novartis, Roche, Sanofi Aventis, and TEVA ratiopharm. Christian Enzinger received funding for travel and speaker honoraria from Bayer, Biogen, Genzyme, Merck, Novartis, Roche, Shire, and Teva Pharmaceutical Industries Ltd./sanofi‐aventis, research support from Biogen, Merck, and Teva Pharmaceutical Industries Ltd./sanofi‐aventis, and served on scientific advisory boards for Bayer, Biogen, Merck, Novartis, Roche, and Teva Pharmaceutical Industries Ltd./sanofi‐aventis. Bettina Heschl received funding for travel and speaker honoraria from Bayer, Biogen, Bristol‐Myers‐Squibb, Janssen, Merck, Novartis, Roche, Sanofi, and Teva. Franziska Di Pauli has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Amgen, Bayer, Biogen, Celgene, Merck, Neuraxpharm, Novartis, Sandoz, Sanofi‐Genzyme, Roche, and Teva. Christiane Gradl has participated in meetings sponsored by or received honoraria (lectures, advisory boards, consultations) and/or travel funding from Alexion, Almirall, Biogen, Bristol‐Myers‐Squibb, D‐Pharma, Horizon, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva‐ratiopharm. Stefan Kalcher declares that there is no conflict of interest. Erich Kvas declares that there is no conflict of interest. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Bayer, Biogen, Biologix, Bionorica, Bristol‐Myers‐Squibb, Eisai, GW Pharma, Horizon, Janssen‐Cilag, MedDay, Merck, Neuraxpharm, Novartis, Octapharma, Roche, Sandoz, Sanofi, TG Pharmaceuticals, TEVA‐ratiopharm, and UCB. His institution has received financial support in the last 12 months by unrestricted research grants (Biogen, Bayer, Bristol‐Myers‐Squibb, Merck, Novartis, Sanofi, and TEVA ratiopharm) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Bristol‐Myers‐Squibb, Merck, Novartis, Octapharma, Roche, Sanofi, and TEVA.
Figures

References
-
- Wiendl H., Gold R., Berger T., et al., “Multiple Sclerosis Therapy Consensus Group (MSTCG): Position Statement on Disease‐Modifying Therapies for Multiple Sclerosis (White Paper),” Therapeutic Advances in Neurological Disorders 14 (2021): 17562864211039648, 10.1177/17562864211039648. - DOI - PMC - PubMed
-
- Guger M., Enzinger C., Leutmezer F., et al., “Effects of Horizontal Versus Vertical Switching of Disease‐Modifying Treatment After Platform Drugs on Disease Activity in Patients With Relapsing‐Remitting Multiple Sclerosis in Austria,” Journal of Neurology 270, no. 6 (2023): 3103–3111, 10.1007/s00415-023-11644-y. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

