Varying Analgesic Effectiveness of Systemic and Central Intrathecal Administration of Cyclooxygenase-2 Inhibitors in Different Phases of Osteoarthritic Pain in Rats
- PMID: 40616299
- PMCID: PMC12228020
- DOI: 10.1002/ejp.70068
Varying Analgesic Effectiveness of Systemic and Central Intrathecal Administration of Cyclooxygenase-2 Inhibitors in Different Phases of Osteoarthritic Pain in Rats
Abstract
Background: Osteoarthritis (OA) contributes to heightened pain perception by disrupting the normal function of peripheral nerves and spinal nociceptive circuits. Although selective cyclooxygenase-2 (COX-2) inhibitors reduce OA-associated pain, the distinct roles of spinal COX-2 and glial cell activity in this context remain poorly defined.
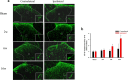
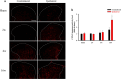
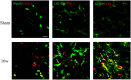
Methods: The effects of two COX-2 inhibitors, etoricoxib and celecoxib, were examined using an anterior cruciate ligament transection (ACLT) rat model of OA. Mechanical allodynia was assessed behaviorally using the von Frey filament test. COX-2 protein expression and glial cell (astrocyte and microglia) activation in the lumbar spinal cord were analysed via immunohistochemistry.
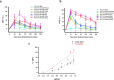
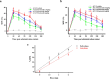
Results: Spinal COX-2 expression was significantly increased, mainly in neurons and astrocytes, at the 16th week after ACLT (p = 0.026). Microglia and astrocytes were activated from the 2nd to 16th week and from the 6th to 16th week after ACLT, respectively. The intrathecal median effective dose (ED50) of COX-2 inhibitors, etoricoxib and celecoxib, required for reducing mechanical allodynia was lower at the 16th week than at the 2nd week after ACLT surgery (p = 0.0448 and 0.046, respectively). In contrast, the oral ED50 values of etoricoxib and celecoxib for relieving mechanical allodynia were slightly higher at the 16th week than at the 2nd week after ACLT surgery (p = 0.097 and 0.227, respectively).
Conclusions: Our study shows that the efficacy of COX-2 inhibitors in ALCT-induced OA rats depends on the timing and route of administration. In the later phase, spinal glia cells exhibited increased activity and elevated COX-2 expression.
Significance: This study characterises the complex mechanisms underlying OA pain, involving both peripheral and central components, and highlights the stage-specific involvement of COX-2, particularly in the spinal cord. It provides experimental evidence linking central COX-2 activity and glial cell responses to OA pain, offering insights into the temporal dynamics of pain processing and guiding the development of therapeutic strategies.
Keywords: anterior cruciate ligament transection; celecoxib; cyclooxygenase‐2; etoricoxib; mechanical allodynia; osteoarthritis.
© 2025 The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Adães, S. , Ferreira‐Gomes J., Mendonça M., Almeida L., Castro‐Lopes J. M., and Neto F. L.. 2015. “Injury of Primary Afferent Neurons May Contribute to Osteoarthritis Induced Pain: An Experimental Study Using the Collagenase Model in Rats.” Osteoarthritis and Cartilage 23: 914–924. - PubMed
-
- Arendt‐Nielsen, L. , Egsgaard L. L., and Petersen K. K.. 2016. “Evidence for a Central Mode of Action for Etoricoxib (COX‐2 Inhibitor) in Patients With Painful Knee Osteoarthritis.” Pain 157: 1634–1644. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

