The Plant Mind: Unraveling Abiotic Stress Priming, Memory, and Adaptation
- PMID: 40616400
- PMCID: PMC12228110
- DOI: 10.1111/ppl.70372
The Plant Mind: Unraveling Abiotic Stress Priming, Memory, and Adaptation
Abstract
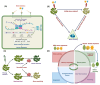
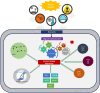
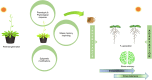
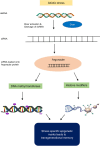
Plants exhibit a remarkable capacity to adapt to recurrent abiotic stresses, prompting a re-evaluation of traditional views on plant responses to environmental challenges. This review explores the intricate mechanisms of stress priming, memory, and adaptation in plants. Specifically, it details the molecular and physiological processes underlying abiotic stress priming, which serve as a gateway to understanding plant memory. Stress priming fosters resilience against diverse stressors through interconnected pathways involving hormone signaling, transcriptional regulation, DNA methylation, histone modifications, and small RNAs. These epigenetic changes orchestrate stress-responsive gene expression and can, in some cases, be passed on to future generations. This review distinguishes between somatic memory, intergenerational effects, and transgenerational inheritance to avoid conceptual overlap. By connecting short-term priming to long-term adaptation and potential heritability, this article proposes a paradigm shift in how plant resilience is understood, with significant implications for crop improvement under climate stress.
Keywords: adaptation; climate change; epigenetic modifications; stress memory; transgenerational inheritance.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Transgenerational Memory of Phenotypic Traits in Plants: Epigenetic Regulation of Growth, Hormonal Balance, and Stress Adaptation.Curr Issues Mol Biol. 2025 May 29;47(6):404. doi: 10.3390/cimb47060404. Curr Issues Mol Biol. 2025. PMID: 40699803 Free PMC article. Review.
-
A review of the potential involvement of small RNAs in transgenerational abiotic stress memory in plants.Funct Integr Genomics. 2024 Apr 11;24(2):74. doi: 10.1007/s10142-024-01354-7. Funct Integr Genomics. 2024. PMID: 38600306 Review.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Coupled dopamine and insulin signaling mediated transgenerational and multigenerational inheritance of adaptive traits in Caenorhabditis elegans upon parental training with Salmonella enterica Serovar Typhi.Microbiol Spectr. 2025 Jul;13(7):e0257524. doi: 10.1128/spectrum.02575-24. Epub 2025 May 22. Microbiol Spectr. 2025. PMID: 40401953 Free PMC article.
-
Chemical application improves stress resilience in plants.Plant Mol Biol. 2025 Mar 19;115(2):47. doi: 10.1007/s11103-025-01566-w. Plant Mol Biol. 2025. PMID: 40105987 Free PMC article. Review.
References
-
- Bruce, T. J. , Matthes M. C., Napier J. A., Bruce T. J. A., and Pickett J. A.. 2007. “Stressful “Memories” of Plants: Evidence and Possible Mechanisms.” Plant Science 173: 603–608. 10.1016/j.plantsci.2007.09.002. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

