Clioquinol as a new therapy in epilepsy: From preclinical evidence to a proof-of-concept clinical study
- PMID: 40616775
- PMCID: PMC12605802
- DOI: 10.1111/epi.18536
Clioquinol as a new therapy in epilepsy: From preclinical evidence to a proof-of-concept clinical study
Abstract
Objective: Drug-resistant epilepsy (DRE) affects >25 million people worldwide and is often associated with neuroinflammation. Increasing evidence links deficiency or malfunctioning of the enzyme phosphoglycerate dehydrogenase (PHGDH), which converts 3-phosphoglycerate to generate serine and the neurotransmitter glycine, with (drug-resistant) epilepsy. Moreover, PHGDH, which is primarily expressed in astrocytes within the brain, has been identified as a critical enzyme in driving macrophage polarization toward an anti-inflammatory state. Hence, PHGDH activators may be beneficial for treating DRE by exhibiting both antiseizure and anti-inflammatory activity. The objective of this study was to identify such PHGDH activators.
Methods: We screened a drug repurposing library for PHGDH activators and assessed their antiseizure and anti-inflammatory properties using various zebrafish and mouse epilepsy models and explored the mechanistic consequences of activating PHGDH in a cell line, in astrocytes, and in zebrafish heads. Finally, we assessed the efficacy of clioquinol as add-on treatment in three severe DRE patients in a clinical open pilot proof-of-concept study.
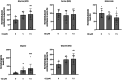
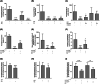
Results: We identified haloquinolines from a drug repurposing library as potent activators of PHGDH. The most promising haloquinoline clioquinol can increase the catalytic activity of PHGDH up to 2.5-fold, thereby increasing de novo glycine biosynthesis and resulting in reduced glutamate levels. Moreover, we show that clioquinol has PHGDH-dependent antiseizure activity as well as anti-inflammatory properties in vivo using various zebrafish and mouse epilepsy models. Finally, we demonstrate the efficacy of clioquinol as add-on treatment in severe DRE patients; two patients showed a 37%-47% reduction in seizure frequency, and all three patients noted a positive impact on quality of life and seizure severity.
Significance: Increasing activity of PHGDH is a promising new approach to treat DRE.
Keywords: de novo glycine biosynthesis; mode of action; therapeutic intervention; translational neuroscience.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
M.M. has served on the advisory board for Merck and has received speaker honoraria from Merck and Biogen. Her institution receives funding from Merck, Australian National Health Medical Research Council, Brain Foundation, Charles and Sylvia Viertel Foundation, and MS Research Australia. L.V.D.B. is head of the scientific advisory board of Augustine Therapeutics (Leuven, Belgium) and part of the investment advisory board of Droia Ventures (Meise, Belgium). L.L. has received grants as well as speaker/consultant honoraria from Zogenix (now part of UCB Pharma), LivaNova, UCB Pharma, Shire, Eisai, Novartis, and Takeda/Ovid. All other authors declare that they have no competing interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
-
- Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34:837–847. - PubMed
-
- Wanigasinghe J, Arambepola C, Sri Ranganathan S, Sumanasena S, Muhandiram EC. The efficacy of moderate‐to‐high dose oral prednisolone versus low‐to‐moderate dose intramuscular corticotropin for improvement of hypsarrhythmia in West syndrome: a randomized, single‐blind, parallel clinical trial. Pediatr Neurol. 2014;51:24–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

