Ascorbic acid deficiency promotes metabolic remodeling and pulmonary fibrosis that leads to respiratory failure in Sod1 and Akr1a double-knockout mice
- PMID: 40616948
- PMCID: PMC12273234
- DOI: 10.1016/j.redox.2025.103749
Ascorbic acid deficiency promotes metabolic remodeling and pulmonary fibrosis that leads to respiratory failure in Sod1 and Akr1a double-knockout mice
Abstract
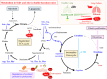
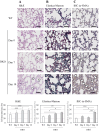
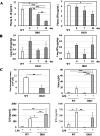
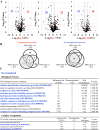
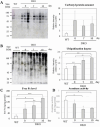
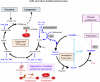
We recently reported that mice with a double knockout (DKO) of Sod1 encoding superoxide dismutase 1 (SOD1) and Akr1a encoding aldehyde reductase survived more than one year when supplemented with ascorbic acid (Asc) (1.5 mg/ml in drinking water), and that the withdrawal of Asc resulted in premature death in only two weeks due to oxidative damage-associated pneumonia. SOD1 is known to disable the radical electrons of superoxide, which suppresses the subsequent formation of highly reactive oxygen species (ROS). Akr1a encodes aldehyde reductase, which catalyzes the biosynthesis of Asc, which is a strong nutritional antioxidant. In this study, we sought to gain insight into the metabolic basis for the progression of respiratory failure in the DKO mice. Pathological examinations have revealed pulmonary damage and the progression of fibrosis caused by an elevation in pulmonary cell death in these mice. Metabolite analyses have shown that substrate compounds catabolized in the tricarboxylic acid cycle are shifted from carbohydrates to amino acids, which leads to polyamine synthesis. While proteins involved in cell polarization, adhesion, and transport are increased in the lungs, showing trends similar to those of activated leukocytes, antioxidative enzymes were characteristically decreased in the lungs. Carbonyl proteins were originally high in the DKO mice but did not increase following Asc withdrawal, which was likely caused by stimulation of the degradation of oxidized proteins through the ubiquitin-proteasome system. It is conceivable that the oxidative insult due to Asc insufficiency under Sod1 deficiency causes protein oxidation followed by degradation, which fuels the tricarboxylic acid cycle. Remodeling the metabolic pathways for amino acid use increases polyamine synthesis, which could stimulate pulmonary fibrosis and lead to respiratory failure.
Keywords: Pneumonia; Proteomics; Superoxide; Tricarboxylic acid cycle; Urea cycle.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest We declare no conflicts of interest between the authors or with any institution in relation to the content of this article.
Figures




Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
References
-
- Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F., Wilcox H.M., Flood D.G., Beal M.F., Brown R.H., Jr., Scott R.W., Snider W.D. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13(1):43–47. doi: 10.1038/ng0596-43. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

