Biological activities of optimized biosynthesized selenium nanoparticles using Proteus mirabilis PQ350419 alone or combined with chitosan and ampicillin against common multidrug-resistant bacteria
- PMID: 40618114
- PMCID: PMC12228243
- DOI: 10.1186/s12934-025-02783-0
Biological activities of optimized biosynthesized selenium nanoparticles using Proteus mirabilis PQ350419 alone or combined with chitosan and ampicillin against common multidrug-resistant bacteria
Abstract
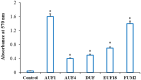
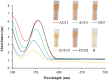
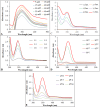
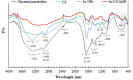
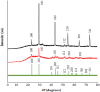
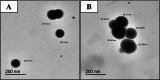
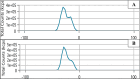
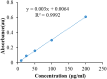
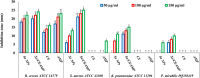
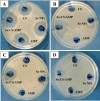
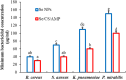
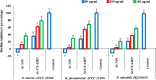
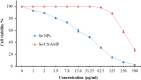
Background: One of the most common issues in the world is bacterial resistance and biofilms, which can prolong the healing period and the need for self-medication. Additionally, they may be linked to unsuccessful therapies, which raises death rates, healthcare expenses, and the need for additional hospitalization. Therefore, to protect the environment and improve human health, there is a need for the creative synthesis of novel antibacterial materials. Proteus mirabilis strain PQ350419 was isolated, identified, and utilized as an efficient bio-nano-factory for biosynthesizing selenium nanoparticles (Se NPs) and optimizing procedures. This study showcases a simple and cost-effective approach for green-synthesizing a selenium/chitosan/ampicillin nanocomposite (Se/CS/AMP) as a novel antibacterial and antibiofilm agent. Several analyses, such as transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, zeta analysis, and ultraviolet-visible (UV-Vis) spectroscopy, were utilized to confirm and characterize the production of Se NPs and Se/CS/AMP. The absorption peaks for Se NPs and Se/CS/AMP were identified to be between 350 and 360 nm. The XRD data revealed the crystalline composition of the Se NPs loaded with CS and AMP. The FTIR spectra confirmed the presence of proteins that act as supporting and binding agents during synthesis. The stability of the prepared nanomaterials is improved by a strong negative surface charge of - 24.27 mV for Se NPs and - 23.92 mV for Se/CS/AMP. The particle sizes of Se NPs and Se/CS/AMP are shown by TEM to be in the ranges of 88-98 nm and 86-129 nm, respectively. Se NPs, either alone or in combination with chitosan (CS) and ampicillin (AMP), exhibited strong antibacterial activity against methicillin-resistant Staphylococcus aureus ATCC 43,300, Bacillus cereus ATCC 14,579, Klebsiella pneumoniae ATCC 11,296, and P. mirabilis PQ350419 in a dose-dependent manner. Compared to Se NPs and the common antibiotic AMP, the Se/CS/AMP combination demonstrated superior antibacterial activity. In comparison to Se NPs (40, 70, 110, and 150 µg/ml, respectively), the nanocomposite produced MIC values of 30, 40, 60, and 100 µg/ml against B. cereus, S. aureus, K. pneumoniae, and P. mirabilis. When compared to untreated cells, treated cells exhibited significant morphological changes and deformities, such as cell wall distortion, the separation of the cell wall from the plasma membrane, the formation of vacuoles, and complete cell lysis, according to TEM ultrastructure studies of bacteria treated with nanocomposite. Se/CS/AMP at 100 µg/ml was sufficient to prevent biofilm formation by up to 50% in S. aureus, K. pneumoniae, and P. mirabilis. The cell viability of the Vero cell line was significantly reduced (p˂0.05) in the cytotoxicity test of Se NPs alone at a concentration of 40.95 ± 2.34 µg/ml, and in its nanocomposite at a concentration of 199.09 ± 2.61 µg/ml. This indicates the nanocomposite's safety by showing its minimal harmful impact on the Vero cell line.
Conclusion: Se/CS/AMP has revealed an antibacterial and antibiofilm agent that could be useful in various industrial, medicinal, and environmental applications. This study introduces a work that presents an alternative, safe, promising, and efficient nanocomposite for treating harmful bacteria in humans and animals. This treatment is based on the synergistic effectiveness of Se NPs, CS, and AMP.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was carried out in accordance with the guidelines and regulations of the scientific research ethics committee, Faculty of Science, Damietta University, Egypt. Written informed consent for enrolment, screening, and specimen collection was obtained from each patient or their parents or guardians. The data of the specimens were not exposed. The cytotoxicity experiments were conducted in compliance with the IACUC (The Institutional Animal Care and Use Committee) statement for using animals in research and teaching by the local ethical committee of AUHA (Al-Azhar University Housing Animals). Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Evaluation of the antibacterial and antibiofilm effect of mycosynthesized silver and selenium nanoparticles and their synergistic effect with antibiotics on nosocomial bacteria.Microb Cell Fact. 2025 Jan 4;24(1):6. doi: 10.1186/s12934-024-02604-w. Microb Cell Fact. 2025. PMID: 39755661 Free PMC article.
-
Combatting biofilm formation of Klebsiella pneumoniae and Bacillus subtilis clinical strains from the oral cavity using biogenic Se-NPs: molecular docking simulation and cytotoxic effects on HepG2 cancer cells.BMC Microbiol. 2025 Jul 21;25(1):446. doi: 10.1186/s12866-025-04142-w. BMC Microbiol. 2025. PMID: 40691756 Free PMC article.
-
Characterization of Silver Nanoparticles Synthesized Using Hypericum perforatum L. and Their Effects on Staphylococcus aureus.Microsc Res Tech. 2025 Aug;88(8):2321-2332. doi: 10.1002/jemt.24862. Epub 2025 Mar 23. Microsc Res Tech. 2025. PMID: 40121669 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Baylay AJ, Piddock LJV, Webber MA. Molecular mechanisms of antibiotic resistance–Part I. Bacterial resistance to antibiotics–from molecules to man. 2019;1–26.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

