LAG3 limits regulatory T cell proliferation in α-synuclein gut-to-brain transmission model
- PMID: 40618163
- PMCID: PMC12228299
- DOI: 10.1186/s12974-025-03502-7
LAG3 limits regulatory T cell proliferation in α-synuclein gut-to-brain transmission model
Abstract
Background: Pathological α-synuclein (α-syn) can spread from the gut to the central nervous system (CNS), with CD4 + T cells playing a key role in this process. Lymphocyte activation gene 3 (LAG3) is involved in intestinal inflammation, regulates CD4 + T cell proliferation and function, and can specifically bind to pathological α-syn during cell-to-cell transmission. However, it remains unclear whether LAG3 is involved in the spread of pathological α-syn from the gut to the brain.
Methods: We utilized LAG3 knockout mice, combined with injection of α-syn preformed fibril (PFF) into the longitudinal and intermediate muscle layers of the pylorus and duodenum to model Parkinson's disease (PD). We used Immunohistochemistry staining, Western Blot, Flow cytometry to detect the changes of TH, α-syn, pro-inflammatory factors, barrier-related proteins and CD4 + T cells differentiation.
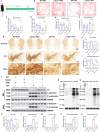
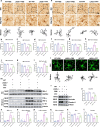
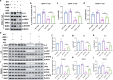
Results: Our results show that LAG3 knockout partially alleviates psychological and behavioral deficits, dopamine system damage, and the gut-to-brain transmission of α-syn, which correlates with enhanced regulatory T cell (Treg) cell proliferation. Furthermore, LAG3 knockout improved intestinal dysfunction and increased the expression of tight junction proteins in both the gut and the blood-brain barrier (BBB). In CD4 + T cells isolated from the spleen, LAG3 knockout suppressed the aggregation of α-syn PFF, thereby inhibiting the toxic T-cell response induced by α-syn PFF. LAG3 deficiency also enhanced the IL-2/STAT5 signaling pathway, which regulates Treg proportions both in vivo and in vitro.
Conclusions: Our findings demonstrated that LAG3 intrinsically limits Treg cell proliferation and function in the environment with pathological α-syn and promotes the gut-to-brain transmission of α-syn.
Keywords: Alpha-synuclein; Gut to brain transmission; LAG3; Parkinson's disease; Preformed fibril; Regulatory T cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Guangzhou Medical University. Competing interests: The authors declare no competing interests.
Figures



References
-
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. - PubMed
-
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in meissner’s and auerbach’s plexuses in cases staged for parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. - PubMed
MeSH terms
Substances
Grants and funding
- 2023B03J0631/Key Technologies Research and Development Program of Guangzhou Municipality
- 2022B1515230004/Basic and Applied Basic Research Foundation of Guangdong Province
- 2023A03J01331/Guangzhou Municipal Science and Technology Bureau's Basic Research Program for the year 2024 jointly funded by the city and universities
- 2023B03003/Xinjiang Uyghur Autonomous Region Key Research and Development Project
- 82101326/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

