p53/PD-L1 co-expression predicts poor prognosis in diffuse large B-cell lymphoma
- PMID: 40618312
- PMCID: PMC12229974
- DOI: 10.1007/s12672-025-03062-5
p53/PD-L1 co-expression predicts poor prognosis in diffuse large B-cell lymphoma
Abstract
Objective: To investigate the clinical characteristics and survival of p53 and Programmed death-ligand 1 (PD-L1) co-expression in diffuse large B-cell lymphoma (DLBCL) patients.
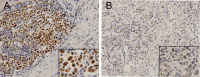
Methods: Immunohistochemistry (IHC) was used to detect the expression of p53 and PD-L1 in tumor cells of 176 patients with DLBCL. Clinical data were retrospectively examined along with long-term follow-up. Correlation between the expression of p53 and PD-L1 and the clinicopathological characteristics of the patients was assessed. Impact of Rituximab (R) on prognosis among DLBCL patients with positive expression of p53 and PD-L1 was evaluated.
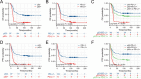
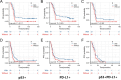
Results: p53 expression, PD-L1 expression, and p53/PD-L1 co-expression were present in 44.9%, 42.0%, and 25.6% of patients with DLBCL, respectively. No significant differences existed in the clinicopathological characteristics between patients with positive and negative p53 expression and between patients with positive and negative PD-L1 expression. However, more co-expression of p53/PD-L1 was observed in patients with non-germinal center B-cell-like (non-GCB) subtypes (p = 0.005). There was a significant positive correlation between p53 and PD-L1 expression (r = 0.273, p < 0.001). Survival analysis indicated that patients exhibiting p53/PD-L1 co-expression had decreased progression-free survival (PFS) and overall survival (OS ) compared to those demonstrating positive expression of either p53 or PD-L1, and in addition, both negative p53/PD-L1 expression. Rituximab failed to substantially modify the prognosis of patients. p53/PD-L1 co-expression is an independent indicator of adverse prognosis.
Conclusions: Co-expression of p53/PD-L1 in DLBCL patients implies an unfavorable prognostic group, which doesn't derive benefit from Rituximab.
Keywords: Diffuse large B-cell lymphoma; PD-L1; Prognosis; Rituximab; p53; p53/PD-L1 co-expression.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Studies involving human participants were approved and reviewed by the Medical Ethics Committee of Tianjin Medical University Cancer Institute & Hospital. The research was carried out following the guidelines of the ethics committee listed in the ethics statement. Consent to participate: Informed consent has been obtained from the patients for the study. Consent to publish: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
