ONO-4578 Plus Nivolumab in Unresectable Advanced or Recurrent Gastric or Gastroesophageal Junction Cancer
- PMID: 40618725
- PMCID: PMC12400061
- DOI: 10.1111/cas.70130
ONO-4578 Plus Nivolumab in Unresectable Advanced or Recurrent Gastric or Gastroesophageal Junction Cancer
Abstract
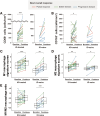
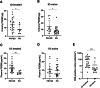
ONO-4578, an EP4 antagonist, alone and combined with nivolumab, showed acceptable safety profiles and signs of antitumor activity in solid tumors. The expansion part examined the safety, preliminary efficacy, and biomarkers of ONO-4578 plus nivolumab in unresectable advanced or recurrent gastric or gastroesophageal junction (G/GEJ) cancer. Patients were enrolled into three groups: with previous immuno-oncology treatment (IO-treated; n = 30), without IO treatment (IO-naive; n = 30), and with UGT1A1 polymorphism (UGT1A1p; n = 6). Treatment-related adverse events (TRAEs) occurred in 46 patients (grade 3-4 in 17), with no grade 5 events reported. We confirmed the tolerability of the treatment in UGT1A1p. Objective response and disease control rates were 10.0% and 73.3%, respectively, in IO-treated and 16.7% and 40.0%, respectively, in IO-naive. Biomarker analysis indicated immune activation in the tumor microenvironment after the treatment. In conclusion, ONO-4578 plus nivolumab showed a manageable safety profile and antitumor activity in G/GEJ cancer. Trial Registration: Japan Registry of Clinical Trials number: jRCT2080223441; ClinicalTrials.gov identifier: NCT03155061.
Keywords: dose‐limiting toxicity; gastric cancer; nivolumab; phase I; prostaglandin E2 receptor EP4.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
All authors declare financial support for the submitted work from Ono. A.K. reports grants from AstraZeneca and MSD; consulting fees from Zymeworks, MSD, Revolution Medicines, and Abbvie; honoraria from Taiho Pharmaceutical, Bristol Myers Squibb (BMS), Merck Serono, Ono, and Daiichi Sankyo; support for attending meetings or travel from Ono and MSD; and participation on an advisory board in Abbvie. K.Ya. reports honoraria from Daiichi Sankyo, Chugai, BMS K.K., Eli Lilly Japan K.K., Taiho, Ono, Takeda, and Merck Biopharma. T.H. reports honoraria from Ono. Y.N. reports grants from Ono, BMS, AstraZeneca, and Daiichi Sankyo; honoraria for lectures, presentations, and speakers bureaus from Yakult Honsha, Taiho, Eli Lilly, Daiichi Sankyo, Ono, and BMS; participation on an advisory board in Daiichi Sankyo. S.B. reports Eisai, Taiho, Nippon Kayaku, MSD, BMS, and Asahi Kasei. T.O. reports grants from Taiho, Chugai, Daiichi Sankyo, Nippon Kayaku, and Kyowa Kirin; honoraria from Astellas, Taiho, Tsumura, Nippon Kayaku, and Takeda. H.Ha. reports grants from ALX Oncology, Amgen, AstraZeneca, Astellas, Bayer, Chugai, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, MSD, Ono, and Taiho; consulting fees for an advisory role from Astellas, Boehringer Ingelheim, and Daiichi Sankyo; honoraria for lectures from Bayer, Chugai, Merck Biopharma, Ono, Taiho, BMS, Daiichi Sankyo, Eli Lilly, Miyarisan, MSD, Takeda, Asahi Kasei, and Yakult. T.E. reports grants from MSD, Daiichi Sankyo, Pfizer, Astellas, Quintiles, Syneos Health, Chugai, Amgen, Ono, Novartis, Astellas Amgen Biopharma, Asahi Kasei, IQVIA, Parexel, Nihon Kayaku, Eli Lilly, Dainippon Sumitomo, Merck Serono, and Bayer; honoraria from Chugai, Daiichi Sankyo, Taiho, BMS, MSD, Ono, Sanofi, Bayer, Merck Serono, and Takeda. T.N. reports honoraria from BMS, Ono, Taiho, Eli Lilly, Daiichi Sankyo, Astellas, and MSD. M.G. reports grants from Chugai, Taiho, and Nippon Kayaku; payment for expert testimony from Tsumura, Daiichi Sankyo, Ono, Taiho, and MSD K.K. E.O. is an editorial member of Cancer Science. N.S. reports honoraria from Chugai, Eli Lilly, Daiichi Sankyo, and MSD Oncology. F.Y., T.Y., K.Yo., and Y.O. are employees of Ono. S.I. reports research grants from Ono, BMS, Chugai, Daiichi Sankyo, Pfizer, Eisai, and Taiho; honoraria from Ono, BMS, and Daiichi Sankyo and is currently an employee of Chugai.
Figures



References
-
- Robert C., Long G. V., Brady B., et al., “Nivolumab in Previously Untreated Melanoma Without BRAF Mutation,” New England Journal of Medicine 372, no. 4 (2015): 320–330. - PubMed
-
- Fehrenbacher L., Spira A., Ballinger M., et al., “Atezolizumab Versus Docetaxel for Patients With Previously Treated Non‐Small‐Cell Lung Cancer (POPLAR): A Multicentre, Open‐Label, Phase 2 Randomised Controlled Trial,” Lancet 387, no. 10030 (2016): 1837–1846. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

