Mesenchymal Stromal Cells from Dental Tissues Demonstrate Neuronal Potential Pre- and Post-Induction
- PMID: 40618736
- PMCID: PMC12503503
- DOI: 10.1159/000547257
Mesenchymal Stromal Cells from Dental Tissues Demonstrate Neuronal Potential Pre- and Post-Induction
Abstract
Introduction: Mesenchymal stromal cells (MSCs) play a crucial role in tissue repair and exhibit anti-inflammatory properties, making them promising for regenerative medicine. Dental tissue-derived MSCs, such as stromal cells from human exfoliated deciduous teeth (SHEDs) and dental pulp stromal cells (DPSCs), express neural markers and hold the potential for treating neurodegenerative diseases. Nonetheless, their relative ability to differentiate into neurons is still unclear. Given their shared embryonic origin, we hypothesised that SHEDs and DPSCs possess similar potential for neuronal differentiation along with intrinsic expression of neuronal markers. The objective of this study was to compare their differentiation abilities under standardised conditions, evaluate neuronal markers pro- and post-neuronal induction, and compare the neuronal differentiation potential of SHEDs and DPSCs.
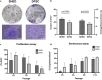
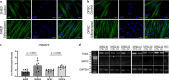
Methods: SHEDs (n = 3) and DPSCs (n = 3) were collected with ethical approval, cultured, and characterised according to established MSC criteria. Clonogenicity, proliferation, senescence, and trilineage differentiation were assessed. Neuronal differentiation was induced for 21 days and evaluated using flow cytometry (SRY-Box Transcription Factor 1 [SOX1], SRY-Box Transcription Factor 2 [SOX2], glial fibrillary acidic protein [GFAP], doublecortin, nestin, CD56, CD146), immunofluorescence for βIII-tubulin, reverse transcription polymerase chain reaction for tubulin 3 (TUB3), and microtubule-associated protein 2 (MAP2).
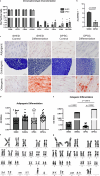
Results: Both SHEDs and DPSCs exhibited MSC characteristics. SHEDs showed higher clonogenicity. Early neuronal markers (e.g., SOX1, nestin, GFAP, βIII-tubulin) were detected pre- and post-induction in both cell types without significant intergroup differences. No significant expression of TUB3 and MAP2 was observed.
Conclusion: SHEDs and DPSCs show comparable neuronal marker expression profiles, suggesting similar early neuronal differentiation potential. These findings support using undifferentiated SHEDs and DPSCs in neuroregenerative strategies, offering cost-effective and safer alternatives to pre-differentiated cells.
Keywords: Dental tissues; Mesenchymal stem cells; Neuronal differentiation; Regenerative medicine; Stem cell differentiation.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7. - PubMed
-
- Shi S, Bartold P, Miura M, Seo B, Robey P, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191–9. - PubMed
-
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

