Proteomics in pancreatic cancer
- PMID: 40619414
- PMCID: PMC12232871
- DOI: 10.1186/s40364-025-00805-y
Proteomics in pancreatic cancer
Abstract
Pancreatic cancer (PC), one of the most aggressive malignancies, is characterized by a dismal prognosis owing to its low early detection rates, rapid progression, frequent postoperative complications, and limited efficacy of conventional oncological therapies. The fact that most patients are diagnosed at advanced stages underscores the critical importance of early detection for the formulation of effective treatment strategies. Despite substantial research efforts, the medical community still lacks consistent and dependable biomarkers for the diagnosis, classification, and prognosis of PC, highlighting the urgent need for innovative and more efficient approaches to identify pancreatic abnormalities at early stages. For decades, mass spectrometry (MS)-based proteomics has been extensively applied in disease diagnostics, mechanistic investigations, and screening of potential drug targets. This review systematically synthesizes recent advancements in clinical proteomic techniques and applications, highlighting significant biomarker discoveries and signal transduction networks associated with PC. By integrating these findings, we provide novel insights into the molecular mechanisms underlying PC development and progression, which may facilitate the identification of new diagnostic biomarkers and therapeutic targets for this disease.
Keywords: Biomarkers; Mass spectrometry; Pancreatic cancer; Pancreatic ductal adenocarcinoma; Proteomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

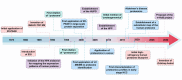

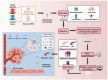


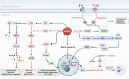
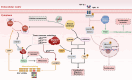
References
-
- Leiphrakpam PD, Chowdhury S, Zhang M, Bajaj V, Dhir M, Are C. Trends in the global incidence of pancreatic Cancer and a brief review of its histologic and molecular subtypes. Journal Gastrointest cancer. 2025;24(1):71. 10.1007/s12029-025-01183-2. - PubMed
-
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;21:2:16022. 10.1038/nrdp.2016.22. - PubMed
-
- Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17(2):108–23. 10.1038/s41571-019-0281-6. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

