Comparative Effectiveness of FF/UMEC/VI and BUD/GLY/FORM in Patients with COPD Stepping Up From Dual Therapy
- PMID: 40619503
- PMCID: PMC12394344
- DOI: 10.1007/s12325-025-03295-4
Comparative Effectiveness of FF/UMEC/VI and BUD/GLY/FORM in Patients with COPD Stepping Up From Dual Therapy
Abstract
Introduction: Recent data suggest differences in effectiveness of single-inhaler triple therapies (SITTs) for patients with chronic obstructive pulmonary disease (COPD); however, data specifically from patients previously treated with dual bronchodilator therapy are lacking. This real-world comparative effectiveness study assessed patients with COPD treated with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) and budesonide/glycopyrrolate/formoterol fumarate (BUD/GLY/FORM) who stepped up from dual therapy.
Methods: This retrospective study used healthcare claims from the Komodo Research database to identify patients with COPD and Medicare Fee-for-Service insurance on dual therapy as their most recent treatment in the 90 days pre-index, stepping up to FF/UMEC/VI or BUD/GLY/FORM between January 1, 2016 and December 31, 2023. Primary outcome was annualized rate of moderate-severe exacerbations per patient-year (PPY) compared using rate ratios (RRs) and 95% confidence intervals (CIs) from Poisson regression models after adjustment with overlap weighting. Secondary outcomes were time to first moderate-severe exacerbation (analyzed as a composite and separately), reported using Kaplan-Meier (KM) analysis and compared using hazard ratios (HRs) with 95% CIs from weighted Cox regression models. All-cause mortality (KM analysis) was included as an exploratory outcome.
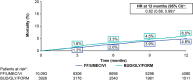
Results: Overall, 10,093 FF/UMEC/VI and 3926 BUD/GLY/FORM patients stepping up from dual therapy were included. Patients stepping up to FF/UMEC/VI experienced an 18% significantly lower rate of moderate-severe COPD exacerbations compared with BUD/GLY/FORM [0.80 vs. 0.98 PPY; RR (95% CI) 0.82 (0.77, 0.88); P < 0.001]. Stepping up to FF/UMEC/VI was also associated with a 14% lower risk of moderate-severe COPD exacerbations [HR (95% CI) 0.86 (0.81, 0.92); P < 0.001] and 18% lower risk of all-cause mortality [HR (95% CI) 0.82 (0.68, 0.99); P = 0.040] at 12 months compared with BUD/GLY/FORM.
Conclusion: In this real-world study, SITT with FF/UMEC/VI was associated with a significantly lower rate and risk of exacerbations compared with BUD/GLY/FORM in patients stepping up from dual therapy.
Keywords: All-cause mortality; Budesonide/glycopyrrolate/formoterol fumarate; Chronic obstructive pulmonary disease; Exacerbations; Fluticasone furoate/umeclidinium/vilanterol; Real-world comparative effectiveness study.
Plain language summary
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease and one of the leading causes of death worldwide. Triple therapy (a combination of three molecules) in a single inhaler is a recommended treatment option for patients with COPD to control COPD attacks; two such single-inhaler triple therapies are available in the United States (US), Europe, and elsewhere; however, information is limited on how they compare in controlling COPD attacks in patients who have previously been treated with dual therapy (a combination of two molecules). This study assessed the effect of treatment with the single-inhaler triple therapies fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) or budesonide/glycopyrrolate/formoterol fumarate (BUD/GLY/FORM) in patients with COPD in the US who had previously used dual therapy as their most recent treatment for 90 days; a healthcare claims database was used to identify the patients. The results of the study suggest that single-inhaler triple therapy with FF/UMEC/VI was associated with a significantly lower rate and risk of COPD attacks compared with BUD/GLY/FORM in patients who had previously used dual therapy. Patients stepping up to triple therapy with FF/UMEC/VI had an 18% lower rate of COPD attacks compared with BUD/GLY/FORM, a 14% lower risk of COPD attacks a year after step up, and an 18% lower risk of death in an exploratory endpoint. These results may help healthcare providers choose the most appropriate treatment for patients with COPD who are inadequately controlled with dual therapy and require treatment with single-inhaler triple therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Jadwiga A. Wedzicha reports grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Novartis, consulting fees from AstraZeneca, EpiEndo Pharmaceuticals, GSK, Gilead, Novartis, Pfizer, Roche, and Empirico, honoraria for lectures, presentations or educational events from AstraZeneca, Boehringer Ingelheim, Glenmark, GSK, Novartis, Recipharm, Roche, and Sanofi, and participation as the data safety monitoring board chair for Virtus. Stephen G. Noorduyn, Valentina Di Boscio, Anurita Majumdar, Rosirene Paczkowski, and Stephen Weng are employees of GSK and/or hold financial equities in GSK. Stephen G. Noorduyn is also a PhD candidate at McMaster University. Olivier Le Rouzic is a principal investigator of CSL Behring and Vertex studies and reports receiving personal fees and/or congress support from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Grifols, GSK, LFB, and Sanofi outside the submitted work. Guillaume Germain and François Laliberté are employees of Groupe d’analyse which received funding from GSK to conduct this study but not for manuscript development. David Mannino is a consultant for AstraZeneca, the COPD Foundation, Genentech, GSK, Regeneron, and UpToDate. David Mannino is also an expert witness on behalf of people suing the tobacco and vaping industries. Ethical Approval: The study complied with all applicable laws regarding patient privacy, as described in the Declaration of Helsinki. No direct patient contact or primary collection of individual human patient data occurred in this study. This study used existing, fully de-identified data that complied with the requirements of the Health Insurance Portability and Accountability Act and the patient(s) cannot be identified, directly or through identifiers. Study results were in tabular form and aggregate analyses that omit patient identification; therefore, informed consent and ethics committee or Institutional Review Board approval were not required.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD: 2025 report. 2025. https://goldcopd.org/2025-gold-report/. Accessed May 16, 2025.
-
- U.S. Food and Drug Administration. TRELEGY ELLIPTA (fluticasone furoate, umeclidinium, and vilanterol). Prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/209482s018lbl.pdf. Accessed May 16, 2025.
-
- U.S. Food and Drug Administration. BREZTRI AEROSPHERE™ (budesonide, glycopyrrolate, and formoterol fumarate). Prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212122s000lbl.pdf. Accessed May 16, 2025.
-
- European Medicines Agency. Trelegy Ellipta. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/trelegy-ellipta. Accessed May 16, 2025.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

