Adaptive Antioxidant Nanomedicines Inhibit Ferroptosis in Renal Tubular Epithelial Cells to Alleviate Diabetic Kidney Disease
- PMID: 40619591
- PMCID: PMC12499382
- DOI: 10.1002/advs.202505168
Adaptive Antioxidant Nanomedicines Inhibit Ferroptosis in Renal Tubular Epithelial Cells to Alleviate Diabetic Kidney Disease
Abstract
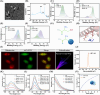
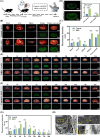
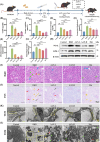
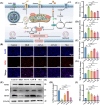
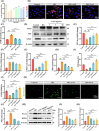
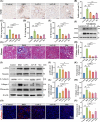
Diabetic kidney disease (DKD) imposes a heavy medical burden worldwide due to the lack of effective treatment. High levels of mtROS and mitochondrial damage in the renal tubules are the initiating and core factors driving the progression of DKD. However, the effectiveness of current antioxidant drugs is greatly limited, mainly due to the difficulty of simultaneously breaching the glomerular barrier and targeting tubular mitochondria, as well as their limited ability to sustain treatment of chronic DKD. Here, this study reports a Se embedded adaptive antioxidant nanodrug (AAN) with negative surface charge and high mitochondrial targeting that can pass through the renal tubules and be highly enriched in the affected renal tubular mitochondria in DKD. AAN can eliminate mtROS to release soluble Se, which is then converted into the key bioactive enzymes -GPX4, effectively inhibiting ferroptosis and protecting mitochondria by exerting adaptive antioxidant effects. In the DKD mouse model, AAN treatment can effectively restore renal function, and the therapeutic effect at a dose of 10 mg kg-1 every 4 days is significantly better than Metformin administered at a dose of 200 mg kg-1 per day. In conclusion, this study provides a promising strategy to enhance the effects of antioxidant therapy to break the pathological barriers in DKD treatment.
Keywords: GPX4; adaptive antioxidant nanodrug; diabetic kidney disease; ferroptosis; mtROS.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cleveland K. H., Schnellmann R. G., Pharmacol. Rev. 2023, 75, 250. - PubMed
-
- Van R. D. H., Bjornstad P., Cherney D. Z. I., Boer I. H. D., Fioretto P., Gordin D., Persson F., Rosas S. E., Rossing P., Schaub J. A., Tuttle K., Waikar S. S., Heerspink H. J. L., Nat. Rev. Nephrol. 2024, 20, 433. - PubMed
-
- Fernandez‐Fernandez B., Ortiz A., Gomez‐Guerrero C., Egido J., Nat. Rev. Nephrol. 2014, 10, 325. - PubMed
-
- Ndumele C. E., Rangaswami J., Chow S. L., Neeland I. J., Tuttle K. R., Khan S. S., Coresh J., Mathew R. O., Baker‐Smith C. M., Carnethon M. R., Despres J.‐P., Ho J. E., Joseph J. J., Kernan W. N., Khera A., Kosiborod M. N., Lekavich C. L., Lewis E. F., Lo K. B., Ozkan B., Palaniappan L. P., Patel S. S., Pencina M. J., Powell‐Wiley T. M., Sperling L. S., Virani S. S., Wright J. T., Rajgopal Singh R., Elkind M. S. V., Circulation 2023, 148, 1606. - PubMed
MeSH terms
Substances
Grants and funding
- 82373871/National Natural Science Foundation of China
- 82100895:81974508/National Natural Science Foundation of China
- 2024JK2114/Key Research and Development Program of Hunan Province
- 2023BEG02038/Key Research Project of Ningxia Hui Autonomous Region of China
- 2023QYJC017/Central South University Research Program of Advanced Interdisciplinary Studies
LinkOut - more resources
Full Text Sources
Medical
