Early Intensive Versus Escalation Approach: Ten-Year Impact on Disability in Relapsing Multiple Sclerosis
- PMID: 40619726
- PMCID: PMC12516235
- DOI: 10.1002/acn3.70131
Early Intensive Versus Escalation Approach: Ten-Year Impact on Disability in Relapsing Multiple Sclerosis
Abstract
Objective: To evaluate the long-term impact of early intensive treatment (EIT) versus escalation (ESC) strategies using high-efficacy disease-modifying therapies (HE-DMTs) on disability progression in relapsing multiple sclerosis (RMS).
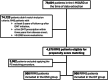
Methods: This observational study included 4878 RMS patients from the Italian Multiple Sclerosis Register. Eligible participants initiated their first disease-modifying therapy (DMT) within 3 years of disease onset and had ≥ 5 years of follow-up with at least three Expanded Disability Status Scale (EDSS) evaluations. Patients were categorized into the EIT group if they started with HE-DMTs and into the ESC group if HE-DMTs were initiated after ≥ 1 year of moderate-efficacy therapy. Propensity score matching was performed to balance baseline characteristics. Outcomes included disability trajectories assessed using linear mixed models for repeated measures and risks of confirmed disability accrual (CDA), progression independent of relapse activity (PIRA), and relapse-associated worsening (RAW) evaluated using Cox proportional hazards models.
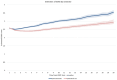
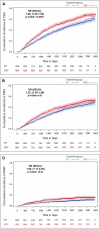
Results: Post-matching analysis of 908 pairs revealed significantly slower disability progression in the EIT group compared to the ESC group. At 10 years, the delta-EDSS difference between groups was -0.63 (95% CI: -0.83 to -0.43; p < 0.0001). ESC was associated with higher risks of CDA (HR 1.36, 95% CI: 1.20-1.54; p < 0.0001), PIRA (HR 1.22, 95% CI: 1.05-1.40; p = 0.0074), and RAW (HR 1.55, 95% CI: 1.17-2.05; p = 0.0021).
Interpretation: EIT significantly reduces long-term disability progression in RMS compared to ESC. These findings underscore the potential of EIT to optimize long-term outcomes in RMS patients.
Keywords: PIRA; disability trajectories; multiple sclerosis.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no conflicts of interest with respect to the contents of the current study, but note that the patients in the study were treated with a number of disease‐modifying drugs and that the authors have received advisory board, membership, speakers honoraria, travel support, research grants, consulting fees, or clinical trial support from the manufacturers of those drugs, including Actelion, Allergan, Almirall, Alexion, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Forward Pharma, Horizon, Ipsen, Medday, Merck Serono, Mylan, Novartis, Sanofi, Roche, Teva, and their local affiliates.
Figures
References
-
- Calabrese M., Preziosa P., Scalfari A., et al., “Determinants and Biomarkers of Progression Independent of Relapses in Multiple Sclerosis,” Annals of Neurology 96, no. 1 (2024): 1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

