Functional study of Phaeodactylum tricornutum Seipin highlights specificities of lipid droplets biogenesis in diatoms
- PMID: 40619984
- PMCID: PMC12329175
- DOI: 10.1111/nph.70350
Functional study of Phaeodactylum tricornutum Seipin highlights specificities of lipid droplets biogenesis in diatoms
Abstract
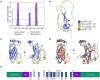
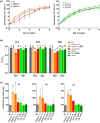
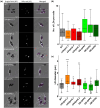
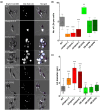
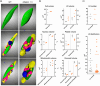
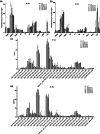
Diatoms are a major phylum of microalgae, playing crucial ecological roles. They derive from secondary endosymbiosis, leading to a complex intracellular architecture. Their ability to store oil in lipid droplets (LDs) upon unfavourable conditions has raised interest for applications, in particular biofuels, yet Lipid Droplet (LD) biogenesis mechanisms in these organisms remain poorly understood. Here, we functionally characterize the homolog of Seipin, a major actor of LD biogenesis, in Phaeodactylum tricornutum. We used an in silico approach to analyze the evolutionary origin of PtSeipin and its specific features. Then, we used a functional genetics approach with a combination of confocal and electronic microscopy and lipidomics to characterize the protein function. We provide evidence that Stramenopiles Seipins were inherited from the host during secondary endosymbiosis. The localization of PtSeipin highlights participation of the plastid's most external membrane in LD biogenesis. Finally, the knock-out (KO) of PtSeipin leads to a strong increase of triacylglycerol (TAG) accumulation, a feature that had not been observed in adipogenic or oleaginous cells and is greatly enhanced following high light exposure. Our results suggest a redirection of lipid fluxes toward TAG synthesis, reduced TAG recycling or a combination of both in PtSeipin KO.
Keywords: Phaeodactylum tricornutum; lipid droplets biogenesis; lipidomics; seipin; triacylglycerol (TAG).
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures



References
-
- Allorent G, Guglielmino E, Giustini C, Courtois F. 2018. Generation of mutants of nuclear‐encoded plastid proteins using CRISPR/Cas9 in the diatom Phaeodactylum tricornutum . In: Maréchal E, ed. Plastids: methods and protocols. New York, NY, USA: Springer US, 367–378. doi: 10.1007/978-1-4939-8654-5_24. - DOI - PubMed
-
- Amari C, Carletti M, Yan S, Michaud M, Salvaing J. 2024. Lipid droplets degradation mechanisms from microalgae to mammals, a comparative overview. Biochimie 227(Pt B): 19–34. - PubMed
MeSH terms
Substances
Grants and funding
- Fonds européen de développement régional (FEDER)
- Commissariat à l'Énergie Atomique et aux Énergies Alternatives
- 91751117/National Natural Science Foundation of China
- ANR-22-CE44-0036-02/Agence Nationale de la Recherche
- R01 GM129325/GM/NIGMS NIH HHS/United States
- ANR-15-IDEX-02/Agence Nationale de la Recherche
- Région Auvergne-Rhône-Alpes
- GRAL Labex ANR-10-LABEX-04/Agence Nationale de la Recherche
- ANR-10-INBS-05-02/Agence Nationale de la Recherche
- PEPR Algadvance A-22-PEBB-0002/Agence Nationale de la Recherche
- 41976119/National Natural Science Foundation of China
- EUR CBS ANR-17-EURE-0003/Agence Nationale de la Recherche
- S-PHAEO/Institut National de Recherche pour l'Agriculture, l'Alimentation et l'Environnement
LinkOut - more resources
Full Text Sources
Research Materials

