Induced Treg-Derived Extracellular Vesicles Suppress CD4+ T-Cell-Mediated Inflammation and Ameliorate Bone Loss During Periodontitis Partly Through CD73/Adenosine-Dependent Immunomodulatory Mechanisms
- PMID: 40620009
- PMCID: PMC12230360
- DOI: 10.1002/jev2.70118
Induced Treg-Derived Extracellular Vesicles Suppress CD4+ T-Cell-Mediated Inflammation and Ameliorate Bone Loss During Periodontitis Partly Through CD73/Adenosine-Dependent Immunomodulatory Mechanisms
Abstract
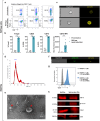
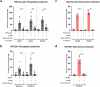
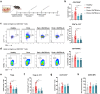
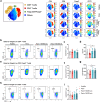
Regulatory T cell (Treg)-derived extracellular vesicles (EVs) represent a contact-independent mechanism by which Tregs suppress dysregulated immune responses. These EVs carry diverse immunomodulatory molecules, including CD73, an ectoenzyme that hydrolyses AMP into adenosine. Adenosine subsequently acts as a potent immunosuppressive mediator that inhibits effector CD4⁺ T cell activation and controls pathological inflammation. Periodontitis is a highly prevalent inflammatory disease characterised by the accumulation of IL-17A-expressing CD4⁺ T cells in response to dysbiotic oral bacterial biofilms, ultimately leading to RANKL-mediated alveolar bone resorption and tooth loss. We tested the hypothesis that CD73⁺ Treg-derived EVs, isolated from Tregs induced with polarising cytokines in the presence of retinoic acid, could limit inflammation and prevent alveolar bone loss in periodontitis. Our findings demonstrate that Tregs induced with polarising cytokines in the presence of retinoic acid express high levels of CD73 and secrete adenosine-producing suppressive CD73+ EVs. Furthermore, local administration of these CD73⁺ Treg-derived EVs in a murine periodontitis model reduced activated CD4⁺ T cell infiltration, decreased IL-17A and RANKL expression, and attenuated osteoclast-mediated alveolar bone loss. In conclusion, retinoic acid-induced Treg-derived EVs suppress CD4⁺ T cell-driven inflammation and ameliorate periodontitis, at least in part through CD73/adenosine-dependent immunomodulatory mechanisms.
Keywords: 5’‐nucleotidase; Treg; adenosine; extracellular vesicles; periodontitis; regulatory T cell; retinoic acid.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- Fondecyt 1210654 (KP-L)/Agencia Nacional de Investigación y Desarrollo (ANID)
- Fondecyt 1220999 (RV)/Agencia Nacional de Investigación y Desarrollo (ANID)
- Ph.D Schollarship 21180841 (CR)/Agencia Nacional de Investigación y Desarrollo (ANID)
- Ph.D Schollarship 21190087 (LG-O)/Agencia Nacional de Investigación y Desarrollo (ANID)
- Ph.D Schollarship 21221750 (AS-C)/Agencia Nacional de Investigación y Desarrollo (ANID)
LinkOut - more resources
Full Text Sources
Research Materials

