Hypotaurine Reduces Glucose-Mediated Vascular Calcification
- PMID: 40620033
- PMCID: PMC12230643
- DOI: 10.1111/apha.70075
Hypotaurine Reduces Glucose-Mediated Vascular Calcification
Abstract
Aim: Vascular calcification (VC), a characteristic feature of peripheral artery disease in patients with diabetes and chronic kidney disease, has been associated with poor prognosis. We hypothesize that hyperglycemia drives VC through alterations in metabolomic and transcriptomic profiles.
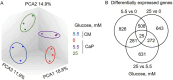
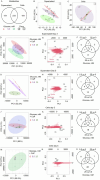
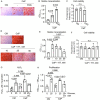
Methods: Human coronary artery smooth muscle cells (SMCs) were cultured with 0, 5.5, and 25 mM glucose under calcifying conditions. Untargeted metabolomic and transcriptomic analyses were performed at different time points. Mitochondrial respiration was examined using Seahorse analysis.
Results: Glucose-treated SMCs promoted extracellular matrix (ECM) calcification in a concentration- and time-dependent manner. The absence of glucose entirely abolished SMC calcification but reduced SMC proliferation in control and calcifying conditions compared to 25 mM glucose. Multi-omics data integration revealed key players from the hypotaurine/taurine metabolic pathway as the center hub of the reconstructed network. Glucose promoted the hypotaurine secretion, while its intracellular abundance was not altered. Blocking hypotaurine production by propargylglycine increased ECM calcification, while hypotaurine treatment prevented it. Furthermore, omics data suggest energy remodeling in calcifying SMCs under hyperglycemia. Calcifying SMCs exhibited decreased oxygen consumption that was partially restored by hypotaurine. Validation of our in vitro models using the murine warfarin model demonstrated reduced hypotaurine/taurine transporter (TAUT) expression in SMCs.
Conclusions: Our multi-omics analysis revealed a role of the hypotaurine/taurine metabolic pathway in glucose-induced SMC calcification. Moreover, our data suggest a glucose-dependent energy remodeling in calcifying SMCs and that increasing glucose concentrations fuel ECM calcification. Our work highlights potential novel therapeutic targets that warrant further investigation in hyperglycemia-dependent in vitro SMC calcification.
Keywords: hypotaurine; metabolomics; transcriptomics; vascular calcification.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
Leon Schurgers receives grants from Gnosis by Lesaffre, Bayer, and Boehringer Ingelheim and is a shareholder of Coagulation Profile. All other authors declare no conflicts of interest.
Figures




References
-
- International Diabetes Federation , “IDF Diabetes Atlas—7th Edition,” (2015), http://www.diabetesatlas.org. - PubMed
-
- National Center for Chronic Disease Prevention and Health Promotion. National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation , “National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States,” (2011), https://www.cdc.gov/diabetes/pubs/pdf/methods11.pdf.
-
- Siitonen O., Suhonen M., and Uusitupa M., “Medial Artery Calcification Predicts Cardiovascular Mortality in Patients With NIDDM,” Diabetes Care 17 (1994): 1252–1256. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

