Defining the Parameters for Sorting of RNA Cargo Into Extracellular Vesicles
- PMID: 40620070
- PMCID: PMC12230367
- DOI: 10.1002/jev2.70113
Defining the Parameters for Sorting of RNA Cargo Into Extracellular Vesicles
Abstract
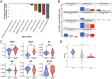
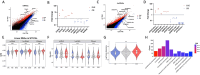
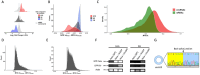
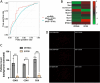
Extracellular vesicles (EVs) are small particles that are released by cells and mediate cell-cell communication by transferring bioactive molecules such as RNA. RNA cargo of EVs, including coding and non-coding RNAs, can change the behaviour of recipient cells, affecting processes including gene expression, proliferation, and Fapoptosis. CircRNAs are stable and resistant to degradation and have been shown to be enriched in EVs. They play key roles in gene regulation and are also emerging as promising biomarkers for disease diagnosis due to their stability and disease-specific expression. Although microRNAs (miRNAs) are the most well studied RNA cargo of EVs, very little is known about the mechanisms of enrichment of circular RNAs (circRNAs) as well as long linear RNAs. Here, we take a comprehensive genome-wide approach to investigate the role of structuredness and shape along with GC%, size, exon count and coding potential, in the sorting and enrichment of circular and long linear RNAs into EVs. We developed a model using these parameters to predict the likelihood of EV packaging of RNA and it was validated by using single molecule RNA imaging of EV bound RNAs. Furthermore, we found that structuredness could explain the relative enrichment of circRNAs over their linear counterparts. These results were validated on existing public databases of circular and linear RNAs in EVs. By identifying and analysing these factors, we aim to better understand the complex mechanisms behind EV-mediated RNA transfer and its impact on cell communication in both health and disease. This mechanistic understanding of RNA enrichment in EVs is crucial for engineering EVs with selective RNA cargo.
Keywords: RNA imaging; SPIRFISH; circRNAs; cis elements; enriched; exosomes; extracellular vesicles; lncRNAs; mRNAs.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
Y.Y. is a named inventor on a patent application (PCT/US2023/020215) for the E3filters used in this study. K.W.W. is or has been an advisory board member of ShiftBio, Exopharm, NeuroDex, NovaDip, and ReNeuron; holds stock options with NeuroDex; and privately consults as Kenneth Witwer Consulting. The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article.
-
Isolation and characterization of bone mesenchymal cell small extracellular vesicles using a novel mouse model.J Bone Miner Res. 2024 Oct 29;39(11):1633-1643. doi: 10.1093/jbmr/zjae135. J Bone Miner Res. 2024. PMID: 39173022 Free PMC article.
-
Systematic proteomic and small RNA profiling of extracellular vesicles from cattle infected with a naturally occurring buparvaquone-resistant strain of Theileria annulata and from uninfected controls.Parasit Vectors. 2025 Jun 10;18(1):221. doi: 10.1186/s13071-025-06834-8. Parasit Vectors. 2025. PMID: 40495253 Free PMC article.
-
Approaches and Challenges in Characterizing the Molecular Content of Extracellular Vesicles for Biomarker Discovery.Biomolecules. 2024 Dec 14;14(12):1599. doi: 10.3390/biom14121599. Biomolecules. 2024. PMID: 39766306 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

