Noninferiority Study Comparing the Efficacy and Safety of a New Hyaluronic Acid (HA) Filler Containing Lidocaine With an Existing HA Filler for the Treatment of Nasolabial Fold Wrinkles: A Randomized, Double-Blind, Split-Face Trial
- PMID: 40620129
- PMCID: PMC12230774
- DOI: 10.1111/jocd.70309
Noninferiority Study Comparing the Efficacy and Safety of a New Hyaluronic Acid (HA) Filler Containing Lidocaine With an Existing HA Filler for the Treatment of Nasolabial Fold Wrinkles: A Randomized, Double-Blind, Split-Face Trial
Abstract
Background: The aesthetic use of hyaluronic acid (HA) has led to the development of various commercial HA fillers. Crosslinked HA dermal fillers are commonly used to treat facial wrinkles. This study demonstrated the non-inferiority of the newly developed hyaluronic acid filler by comparing its efficacy and safety to that of the existing hyaluronic acid filler.
Methods: This 48-week study was designed as a randomized, double-blind, split-face trial. Subjects who met the inclusion and exclusion criteria had a total of four follow-up visits after receiving a dermal filler in the nasolabial folds. Efficacy was assessed using the Wrinkle Severity Rating Score (WSRS) and the Global Aesthetic Improvement Scale (GAIS). Safety was determined through the evaluation of laboratory tests, monitoring adverse events, and verifying vital signs.
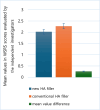
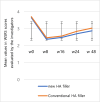
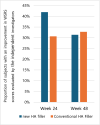
Results: WSRS measured at week 24 of the follow-up visit showed no statistically significant difference, with scores of 2.02 ± 0.71 in the new filler group and 2.27 ± 0.68 in the existing filler group. Even at the 48-week follow-up visit, the improvement rate in the Wrinkle Severity Rating Scale (WSRS) of more than 1 point did not show a statistically significant difference between the new filler group and the existing filler group. The GAIS scores at each follow-up visit showed no statistically significant differences between the two groups. The most frequently reported symptoms related to the test device during the 48-week trial were pain and swelling. However, these symptoms were not statistically significant when compared to the control group from a treatment perspective.
Conclusions: In a 48-week clinical trial aimed at treating nasolabial fold (NLF) wrinkles, the new hyaluronic acid (HA) filler demonstrated efficacy and safety comparable to that of existing control filler. As a result, the new HA filler is both an effective and safe option for improving NLF wrinkles.
Trial registration: This study was registered with ClinicalTrials.gov under the registration number NCT06310863.
Keywords: dermal fillers; hyaluronic acid; nasolabial fold.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- In Cheon H., Kim J. H., Kim B. J., and Lee Y. W., “Efficacy and Safety of a New Hyaluronic Acid Filler for Nasolabial Folds: A 52‐Week, Multicenter, Randomized, Evaluator/Subject‐Blind, Split‐Face Study,” Journal of Cosmetic Dermatology 20, no. 5 (2021): 1467–1473, 10.1111/jocd.13773. - DOI - PubMed
-
- Wang F., Garza L. A., Kang S., et al., “In Vivo Stimulation of De Novo Collagen Production Caused by Cross‐Linked Hyaluronic Acid Dermal Filler Injections in Photodamaged Human Skin,” Archives of Dermatology 143 (2007): 155–163. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

