SiglecF Expressing Neutrophils Exacerbate Th17-Mediated Autoimmune Neuroinflammation
- PMID: 40620419
- PMCID: PMC12226254
- DOI: 10.4110/in.2025.25.e19
SiglecF Expressing Neutrophils Exacerbate Th17-Mediated Autoimmune Neuroinflammation
Abstract
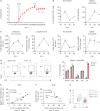
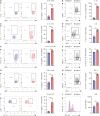
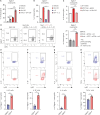
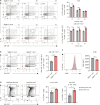
Multiple sclerosis is an autoimmune disease characterized by numerous immune cells, including neutrophils, infiltrating the central nervous system. Previous reports point to a complex role for neutrophils in experimental autoimmune encephalomyelitis (EAE), where their heterogeneity remains poorly understood. In this study, we identified a unique population of neutrophils expressing SiglecF in the brain during EAE that can influence T cell activity. These neutrophils produced elevated levels of Th17-polarizing cytokines, including IL-6, IL-1β, IL-23, and TNF-α, both in vivo and in vitro. Consistent with this cytokine profile, co-culturing SiglecF+ neutrophils with CD4+ T cells promoted Th17 and GM-CSF+ pathogenic Th17 differentiation and proliferation while reducing regulatory T cell numbers. Depleting SiglecF+ neutrophils with anti-SiglecF Abs reduced the severity of EAE, decreased the Th17 population, and increased the regulatory T cell population in the brain. These findings suggest that SiglecF+ neutrophils promote autoimmune neuroinflammation by reinforcing pathogenic autoreactive Th17 cell responses.
Keywords: Autoimmune disease; Experimental autoimmune encephalomyelitis; Neutrophil heterogeneity; SiglecF+ neutrophil; Th17 cells.
Copyright © 2025. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

References
-
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. - PubMed
-
- Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–768. - PubMed
-
- Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7:310ra166 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

