Deep learning to predict progression independent of relapse activity at a first demyelinating event
- PMID: 40620473
- PMCID: PMC12226453
- DOI: 10.1093/braincomms/fcaf243
Deep learning to predict progression independent of relapse activity at a first demyelinating event
Abstract
Progression independent of relapse activity is the main cause of irreversible disability in multiple sclerosis and is strongly associated with older age at symptom onset. Early and accurate prediction, at symptom onset, of which patients are at highest risk of progression independent of relapses, is an unmet need. This study aimed to develop a deep learning survival model using only routine MRI acquired at the first demyelinating attack to predict the risk of progression independent of relapses, and assess its ability to improve classical age-adjusted predictions. We analysed a prospective cohort of patients under 50, clinically assessed within three months of symptom onset, with available MRI (T1- and T2-Fluid-Attenuated Inversion Recovery sequences). An independent early multiple sclerosis cohort (≤1 year from symptom onset) from the Multiple Sclerosis Partners Advancing Technology and Health Solutions database (N = 32) was used for external validation. Patients were assessed for progression independent of relapse activity, defined as a 6-month confirmed increase in the Expanded Disability Status Scale without relapses. Our deep learning model used EfficientNet to estimate the cumulative probability of progression independent of relapses at 1-year intervals. We employed 5-fold cross-validation for model training and testing, assessing performance with the time-dependent concordance index. We also investigated the optimal cumulative probability threshold for binary risk stratification. The model's ability to improve a classical Cox regression model was evaluated. Additionally, we identified brain regions most relevant to deep learning-based progression independent of relapse activity predictions using an interpretability algorithm. A total of 259 patients were evaluated, 58 (22%) of whom experienced at least one event of progression independent of relapse activity over a median follow-up of 4.2 years. The deep learning model demonstrated high performance (time-dependent concordance index = 0.72) with an accuracy of 78% in the original cohort and 72% in the external cohort for predicting the risk of progression independent of relapse activity. Incorporating the deep learning-derived cumulative probability of progression independent of relapses significantly improved an age-adjusted Cox regression model, raising Harrell's C index from 0.62 to 0.74. Interpretability revealed the frontoparietal cortex as a key region in predicting progression independent of relapse activity. In conclusion, our deep learning survival model, based on routine MRI at the first demyelinating attack, can accurately identify patients at high risk of progression independent of relapses and may serve as a valuable tool in clinical practice.
Keywords: brain MRI; deep learning; multiple sclerosis; predictive model; progression independent of relapse activity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
L.C., F.A.-S., J.C., A.Z., I.G., L.M., M.A. and A.O. have nothing to disclose. D.P. has received a research contract with Biogen Idec, and a grant from Instituto Salud Carlos III (PI18/00823). A.C.-C. has received a grant from Instituto de Salud Carlos III, Spain; JR19/00007. G.A. has received compensation for consulting services, participation in advisory boards or speaking honoraria from Merck, Roche and Horizon Therapeutics; and travel support for scientific meetings from Novartis, Roche, and ECTRIMS. G.A. is editor for Europe of the Multiple Sclerosis Journal—Experimental, Translational and Clinical; a member of the executive committee of the International Women in Multiple Sclerosis (iWiMS) network, and a member of the European Biomarkers in MS (BioMS-eu) consortium steering committee. She is a recipient of grants PI19/01590 and PI22/01570, awarded by the Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación de España. Á.V.-J. has engaged in consulting and/or participated as speaker in events organized by Roche, Novartis, Merck and Sanofi. M.C. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merk Serono, Biogen-Idec, Teva Pharmaceuticals, Sanofi-Aventis and Novartis. B.R.-A. has received honoraria for consulting services from Wellspect. C.N. has received funding for travel from Biogen Idec and F. Hoffmann-La Roche, Ltd. and speaker honoraria from Novartis. C.A. has received speaking honoraria from Novartis, Biogen and Stendhal. J.R. has received speaking honoraria and personal compensation for participating on Advisory Boards from Biogen-Idec, Genzyme, Merck-Serono, Mylan, Novartis, Roche, Teva and Sanofi-Aventis. J.S.-G. serves as co-Editor for Europe on the editorial board of Multiple Sclerosis Journal and as Editor-in-Chief in Revista de Neurología, receives research support from Fondo de Investigaciones Sanitarias (19/950) and has served as a consultant/speaker for Biogen, Celgene/Bristol Meyers Squibb, Genzyme, Novartis and Merck. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono, Genzyme, Hoffmann-La Roche, Immunic, Janssen Pharmaceuticals, Medday, Merck, Mylan, Nervgen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF and NMSS. A.R. serves/ed on scientific advisory boards for Novartis, Sanofi-Genzyme, Synthetic MR, Roche, Biogen, Bayer and OLEA Medical; has received speaker honoraria from Bayer, Sanofi Genzyme, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche, and Biogen; and is CMO and co-founder of TensorMedical. M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis and Teva Pharmaceuticals. M.T. is former co-editor of Multiple Sclerosis Journal. X.L. is currently being supported by the ICREA Academia Program. He has also received support from the PID2020-114769RBI00 and the PID2023-146187OB-I00 projects funded by the Ministerio de Ciencia, Innovación y Universidades. C.T. is currently being funded by a Miguel Servet contract, awarded by the Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación de España (CP23/00117). She has also received a 2020 Junior Leader La Caixa Fellowship (fellowship code: LCF/BQ/PI20/11760008), awarded by ‘la Caixa’ Foundation (ID 100010434), a 2021 Merck’s Award for the Investigation in MS, awarded by Fundación Merck Salud (Spain), 2021 and 2024 Research Grants (PI21/01860 and PI24/01277) awarded by the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación de España, and a FORTALECE research grant (FORT23/00034) also by the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación de España. In 2015, she received an ECTRIMS Post-doctoral Research Fellowship and has received funding from the UK MS Society. She is a member of the Editorial Board of Neurology Journal and Multiple Sclerosis Journal. She has also received honoraria from Roche, Novartis, Merck, Sanofi, Johnson and Johnson, and Bristol Myers Squibb, and is a steering committee member of the O’HAND trial and of the Consensus group on Follow-on DMTs.
Figures


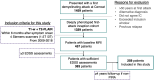

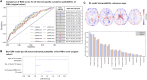
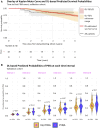
References
-
- Portaccio E, Bellinvia A, Fonderico M, et al. Progression is independent of relapse activity in early multiple sclerosis: A real-life cohort study. Brain. 2022;145(8):2796–2805. - PubMed
-
- Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30(7):767–784. - PubMed
LinkOut - more resources
Full Text Sources
