Preoperative Prediction of a Rare and Highly Aggressive Subtype of Hepatocellular Carcinoma Based on Multimodal Imaging and Clinical Indicators
- PMID: 40620669
- PMCID: PMC12227323
- DOI: 10.2147/JHC.S533963
Preoperative Prediction of a Rare and Highly Aggressive Subtype of Hepatocellular Carcinoma Based on Multimodal Imaging and Clinical Indicators
Abstract
Purpose: To develop and validate a reliable preoperative non-invasive diagnostic model for dual-phenotype hepatocellular carcinoma (DPHCC) by integrating multimodal imaging and clinical indicators, thereby facilitating clinical decision-making.
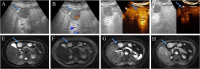
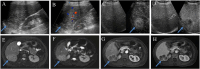
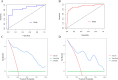
Patients and methods: 222 pathologically confirmed patients (61 with DPHCC, 161 with non-DPHCC) were retrospectively enrolled in this study and randomly assigned to training and validation cohorts in an 8:2 ratio. Serological and multimodal imaging characteristics were analyzed. Univariate and multivariate logistic regression analyses identified independent DPHCC predictors and built a nomogram. Model performance and clinical utility were assessed by receiver operating characteristic (ROC) and decision curve analysis (DCA) curve respectively. The calibration curve was used to verify the model. Recurrence-free survival (RFS) was assessed using Kaplan-Meier and Log rank tests.
Results: In multivariate analysis, age (OR=0.91; P < 0.001), LDH (OR=1.03; P=0.002), PT (OR=0.14; P < 0.001), AFP (OR=4.04; P=0.019), Adler grade (OR=0.17; P=0.037), non-enhancing area (OR=8.30; P=0.004), arterial phase hyperenhancement (OR=0.12; P=0.015) and enhancing capsule (OR=0.32; P=0.04) were independent predictors of DPHCC. The nomogram achieved a robust predictive performance with C-index (0.92 vs 0.87) and accuracy (0.87 vs 0.86) in the training and validation cohorts. In addition, the calibration curve and DCA also showed good model performance. DPHCC patients had significantly lower RFS than non-DPHCC patients (P = 0.037).
Conclusion: A nomogram was established for non-invasive prediction of DPHCC risk utilizing multimodal imaging combined with clinical indicators to help achieve personalized treatment.
Keywords: combined model; contrast enhanced magnetic resonance imaging; contrast enhanced ultrasound; dual-phenotype hepatocellular carcinoma; hepatocellular carcinoma; nomogram.
© 2025 Chen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Differentiation of dual- and non-dual-phenotype hepatocellular carcinoma based on contrast-enhanced computed tomography and patient characteristics.Quant Imaging Med Surg. 2025 Aug 1;15(8):7146-7154. doi: 10.21037/qims-24-1168. Epub 2025 Jul 23. Quant Imaging Med Surg. 2025. PMID: 40785933 Free PMC article.
-
[Study of a nomogram model of gadoxetate disodium-enhanced magnetic resonance imaging for the preoperative diagnosis of proliferative hepatocellular carcinoma and its value].Zhonghua Gan Zang Bing Za Zhi. 2025 Mar 20;33(3):227-236. doi: 10.3760/cma.j.cn501113-20240509-00246. Epub 2024 Nov 20. Zhonghua Gan Zang Bing Za Zhi. 2025. PMID: 40274548 Chinese.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2022 Sep 2;9(9):CD013483. doi: 10.1002/14651858.CD013483.pub2. Cochrane Database Syst Rev. 2022. PMID: 36053210 Free PMC article.
References
-
- Wang QB, Li J, Zhang ZJ, et al. The effectiveness and safety of therapies for hepatocellular carcinoma with tumor thrombus in the hepatic vein, inferior vena cave and/or right atrium: a systematic review and single-arm meta-analysis. Expert Rev Anticancer Ther. 2025;25(5):561–570. doi: 10.1080/14737140.2025.2489651 - DOI - PubMed
LinkOut - more resources
Full Text Sources

