Platelet Membrane-Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles as a Targeting Drug Delivery System for Multidrug-Resistant Breast Cancer
- PMID: 40620683
- PMCID: PMC12229239
- DOI: 10.2147/IJN.S517753
Platelet Membrane-Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles as a Targeting Drug Delivery System for Multidrug-Resistant Breast Cancer
Abstract
Introduction: Paclitaxel (PTX), widely used chemotherapeutic agent, is limited by poor solubility, P-glycoprotein (P-gp) mediated efflux, and non-specific toxicity. To overcome these challenges, we developed a triple-functionalized nanocarrier system incorporating poly(lactide-co-glycolide) (PLGA)-based nanoparticles (PNs), D-α-tocopheryl polyethylene glycol succinate (TPGS) for P-gp inhibition, and platelet membrane (PM) coating for targeted tumor delivery.
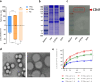
Methods: The PM-coated TPGS-modified PNs with PTX (PTPNs) was characterized by particle size analysis, transmission electron microscopy (TEM), and protein assay to confirm PM coating. In vitro drug release studies were conducted under acidic conditions mimicking the tumor microenvironment. Cellular assays were performed to evaluate cytotoxicity and drug efficacy in multidrug-resistant MCF-7/ADR cells. In vivo biodistribution and xenograft studies assessed tumor accumulation and therapeutic outcomes.
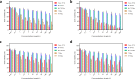
Results: PTPNs exhibited a particle size of 221 ± 2 nm with a PDI of 0.090 ± 0.020 and a zeta potential of -30.5 ± 0.3 mV, indicating a homogeneous particle distribution and successful PM coating. The optimal PM-to-PLGA weight ratio was determined to be 0.005, which ensured structural stability and uniform coating in physiological conditions. Sustained PTX release was observed in acidic conditions, mimicking the tumor microenvironment. Cellular assays showed a 17-fold reduction in PTX IC50 in MCF-7/ADR cells compared to free PTX, attributed to the synergistic effects of TPGS-mediated P-gp inhibition and PM-based tumor targeting. In vivo, PTPNs demonstrated enhanced tumor accumulation and significantly reduced tumor burden, with final tumor volume 2.6-fold lower than that of TPNs and 3.6-fold lower than that of the PTX commercial product (Taxol®)-treated group. Tumor necrosis factor-α (TNF-α) levels were also reduced, reflecting decreased tumor-promoting cytokine activity.
Conclusion: The PTPNs enhanced PTX delivery by improving tumor specificity, overcoming multidrug resistance, and reducing systemic toxicity. These results suggested the potential of this biomimetic approach to advance cancer therapy.
Keywords: Poly(lactide-co-glycolide); cancer therapy; multidrug resistance; nanoparticles; paclitaxel; platelet membrane.
© 2025 Song et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

