Diagnostic yield of 1000 trio analyses with exome and genome sequencing in a clinical setting
- PMID: 40620702
- PMCID: PMC12226303
- DOI: 10.3389/fgene.2025.1580879
Diagnostic yield of 1000 trio analyses with exome and genome sequencing in a clinical setting
Abstract
Introduction: A trio analysis refers to the strategy of exome or genome sequencing of DNA from a patient, as well as parents, in order to identify the genetic cause of a disorder or syndrome.
Methods: During the last 10 years, we have successfully applied exome or genome sequencing and performed trio analysis for 1,000 patients.
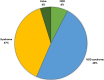
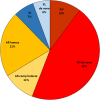
Results: Overall, 39% of the patients were diagnosed, with the detection of causative variant(s). The variants were located in 308 different genes. Autosomal dominant de novo variants were detected in 46% of the solved cases. Detection rates were highest in patients with a syndromic neurodevelopmental disorder (46%) and in patients with known consanguinity (59%). Even for patients previously analyzed as singletons, using a pre-defined gene panel, a consecutive trio analysis resulted in the detection of a causative variant in 30%.
Discussion: A major advantage of trio analysis is the immediate identification of de novo variants as well as confirmation of compound heterozygosity. Additionally, inherited variants from a healthy parent can be dismissed as non-disease causing. The trio strategy enables analysis of a high number of genes-or even the whole genome-simultaneously. The strengths of a trio analysis, in combination with analysis of genome sequence data, allows for the detection of a wide range of genetic aberrations. This enables a high diagnostic yield, even in previously analyzed patients. Our current protocol for trio analysis is based on genome sequencing data, which allows for simultaneous detection of single nucleotide variants, insertion/deletions, structural variants, expanded short tandem repeats, as well as a copy number analysis corresponding to an array-CGH, and analysis regarding SMN1 gene copies.
Keywords: NDD; de novo; exome; genome; syndrome; trio analysis.
Copyright © 2025 Malmgren, Kvarnung, Gustafsson, Anderlid, Arthur, Carlsten, De Geer, Ehn, Grigelioniené, Hammarsjö, Helgadottir, Hellström-Pigg, Iwarsson, Kuchinskaya, Lindelöf, Mannila, Nilsson, Pettersson, Rudd, Sahlin, Tesi, Tham, Thonberg, Westenius, Winberg, Winerdal, Nordenskjöld, Johansson-Soller, Wirta, Nordgren, Lindstrand and Lagerstedt-Robinson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- American psychiartic association (2022). Diagnostic and statistical manual of mental disorders. Fifth Edition.
-
- Bergstrand S., Böhm S., Malmgren H., Norberg A., Sundin M., Nordgren A., et al. (2020). Biallelic mutations in WRAP53 result in dysfunctional telomeres, Cajal bodies and DNA repair, thereby causing Hoyeraal-Hreidarsson syndrome. Cell Death Dis. 11 (4), 238. 10.1038/s41419-020-2421-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

