Induction of hemagglutinin stalk reactive antibodies by the administration of a live-attenuated influenza virus vaccine in children
- PMID: 40620902
- PMCID: PMC12226393
- DOI: 10.1016/j.isci.2025.112893
Induction of hemagglutinin stalk reactive antibodies by the administration of a live-attenuated influenza virus vaccine in children
Abstract
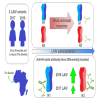
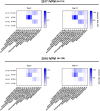
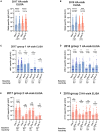
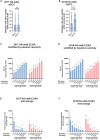
Early life exposures to influenza viruses may imprint a hemagglutinin group-specific signature on immunity that impacts future responses to infection or vaccination. We assessed the administration of a live attenuated influenza virus (LAIV) vaccine in children. Two LAIV formulations (2016-17 and 2017-18) containing distinct H1N1 components were used. Modest boosting of pre-existing serum stalk reactive titers and enhancement of functional antibody-dependent cellular cytotoxicity activity (ADCC) was observed. The magnitude of stalk antibody induction in children naive to influenza A viruses was low; however, LAIV induced de novo stalk antibodies, increasing the number of children seropositive to both group 1 (G1) and group 2 (G2) influenza viruses. The 2018 LAIV formulation, containing an updated H1N1 component, induced higher stalk reactive antibodies with strong ADCC effector functions to the G1 stalk. No significant changes were detected in NA-reactive antibodies in serum or in stalk- or NA-secretory IgA (sIgA) in oral fluid.
Keywords: Immunity.
© 2025 The Authors.
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines on which FK is listed as an inventor. The Krammer laboratory has received support for influenza virus research in the past from GSK and is currently receiving support from Dynavax. FK is currently consulting for GSK, Third Rock Ventures, Pfizer, and Avimex.
Figures
Similar articles
-
Interactions between live attenuated influenza vaccine and nasopharyngeal microbiota among children aged 24-59 months in The Gambia: a phase 4, open-label, randomised controlled trial.Lancet Microbe. 2025 Mar;6(3):100971. doi: 10.1016/j.lanmic.2024.100971. Epub 2025 Jan 17. Lancet Microbe. 2025. PMID: 39832517 Clinical Trial.
-
Binding antibody titers against the hemagglutinin and neuraminidase correlate with protection against medically attended influenza A and B disease.J Virol. 2025 Jun 17;99(6):e0039125. doi: 10.1128/jvi.00391-25. Epub 2025 May 13. J Virol. 2025. PMID: 40358209 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Influenza vaccine for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2018 Jun 26;6(6):CD002733. doi: 10.1002/14651858.CD002733.pub3. Cochrane Database Syst Rev. 2018. PMID: 29943802 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

