C3d-targeted complement inhibitors to correct complement dysregulation in aHUS patients
- PMID: 40621468
- PMCID: PMC12226290
- DOI: 10.3389/fimmu.2025.1620996
C3d-targeted complement inhibitors to correct complement dysregulation in aHUS patients
Abstract
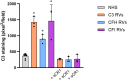
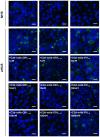
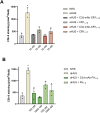
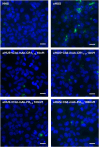
Atypical hemolytic uremic syndrome (aHUS) is a rare and severe thrombotic microangiopathy caused by genetic or acquired abnormalities leading to activation of the complement alternative pathway on cell surfaces. This process leads to endothelial dysfunction and microvascular thrombosis. The introduction of anti-C5 antibodies has dramatically improved aHUS prognosis; however, these treatments require regular intravenous infusions and block systemic complement activity, exposing the patient to risk of infections. Recently complement inhibitors have been developed to selectively bind injury-associated target molecules, thereby concentrating the drug at specific cellular or tissue sites while preserving systemic complement function. This study evaluated the local complement inhibitory activity of new molecules that exploit the natural localization of C3d at complement activation sites on cells: namely the anti-C3d monoclonal antibody 3d8b conjugated with the first 10 or 17 short consensus repeats (SCRs) of complement receptor 1 (CR11-10 and CR11-17, respectively) or the first 5 SCRs of complement factor H (FH1-5). To this purpose we tested their capability to block C3 deposition and C5b-9 formation on microvascular endothelial cells (HMEC-1) exposed to serum from patients with aHUS. We also assessed their ability to prevent loss of anti-thrombogenic properties in HMEC-1 pre-exposed to aHUS serum and then perfused with control blood. We demonstrate that anti-C3d-antibody conjugated with CR11-10, or CR11-17, or FH1-5 effectively prevented aHUS serum-induced complement activation on HMEC-1, outperforming their non-targeted soluble counterparts. The efficacy of C3 convertase inhibition varied depending on the complement inhibitory component (CR11-17 > CR11-10 > FH1-5). However, all the inhibitors successfully blocked C5 convertase activity and eliminated the pro-thrombogenic effects of aHUS patients' serum. These findings support the potential of tissue-targeted complement inhibition as a novel, non-systemic therapeutic strategy for aHUS and other diseases characterized by localized complement dysregulation.
Keywords: aHUS; complement inhibitors; complement system; endothelial cell (EC); thrombus formation.
Copyright © 2025 Guaschino, Santarsiero, Gastoldi, Thurman, Holers, Violette, Liu, Fahnoe, Guarinoni, Benigni, Remuzzi, Noris and Aiello.
Conflict of interest statement
MN has received honoraria from Alexion Pharmaceuticals, Sobi and Novartis for giving lectures, and for participating in advisory boards. GR has had consultancy agreement with Alexion Pharmaceuticals Inc., Janssen Pharmaceutical, Akebia Therapeutics, Biocryst Pharmaceuticals, Menarini Ricerche SpA, AstraZeneca; speaker honoraria/travel reimbursement from Boehringer Ingelheim, Novartis. VMH and JT hold equity in Q32 Bio and have received consulting income. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Noris M, Bresin E, Mele C, Remuzzi G. Genetic atypical hemolytic-uremic syndrome. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® . University of Washington, Seattle, Seattle (WA) (1993). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK1367/. - PubMed
-
- Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. (2020) 97:1287–96. doi: 10.1016/j.kint.2020.01.035 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

