Comparison of Omicron and respiratory virus infections in pediatric liver transplantation: Impact of perioperative identification status
- PMID: 40621858
- PMCID: PMC12239767
- DOI: 10.1080/21505594.2025.2525929
Comparison of Omicron and respiratory virus infections in pediatric liver transplantation: Impact of perioperative identification status
Abstract
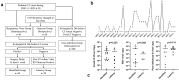
Peri-operative respiratory virus (RV) infection is critical in paediatric liver transplantation. However, it has been inadequately studied, especially in terms of the clinical latency of infection (incubation period) and Omicron variant infection. Herein, we compared the infection profile of common RVs and Omicron variants in paediatric liver transplantation, aiming to identify the association of virus infection with outcomes. The Omicron cohort was designed prospectively, and the RV cohort was retrospective. Survival outcomes, medical resources, and major complications were compared. Risk factors associated with peri-operative mortality were investigated using regression analysis. We enrolled 649 paediatric liver transplantation patients, including 28 Omicron and 61 RV infections. The 1-y overall survival was 97.7 ± 0.6% for the non-infected group, and 93.4 ± 3.2% for the RV group (p = 0.092). No death occurred in the Omicron group. Mortality was higher in the clinical latency infection group compared with that in the non-infected group (13.8% vs. 1.4%, p = 0.002). Latent RV infection (hazard ratio (HR) = 6.323, 95% confidence interval (CI): 1.374-29.087), Multi‑drug resistance organism pneumonia (HR = 7.177, 95% CI: 1.817-28.350), infectious shock (HR = 4.284, 95% CI: 0.995-18.442) and blood loss (HR = 3.209, 95% CI: 1.166-8.833) were independent risk factors for peri-operative mortality. In conclusion, pre-transplant viral screening is fundamental to paediatric liver transplantation. Peri-operative Omicron infection might be controllable, while RV infection led to more complications compared with those in the non-infected group. Clinical latency infection is the key risk for paediatric liver transplantation mortality.
Keywords: COVID-19; Omicron; Pediatric liver transplantation; clinical latency infection; respiratory syncytial virus; respiratory virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Co-infections during SARS-CoV-2 infection in hematologic patients and cell therapy recipients in the omicron era: a Spanish hematopoietic stem cell transplantation and cell therapy group study.BMC Infect Dis. 2025 Jul 25;25(1):944. doi: 10.1186/s12879-025-11302-w. BMC Infect Dis. 2025. PMID: 40713537 Free PMC article.
-
Nirmatrelvir combined with ritonavir for preventing and treating COVID-19.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD015395. doi: 10.1002/14651858.CD015395.pub3. Cochrane Database Syst Rev. 2023. PMID: 38032024 Free PMC article.
-
Household clusters of SARS-CoV-2 Omicron subvariants contemporaneously sequenced from dogs and their owners.mSphere. 2025 Jul 29;10(7):e0007425. doi: 10.1128/msphere.00074-25. Epub 2025 Jul 2. mSphere. 2025. PMID: 40600703 Free PMC article.
-
Association Between Mortality and Detection of Respiratory Viruses in Children During the Peri-Liver Transplant Period.Pediatr Infect Dis J. 2025 Mar 12;44(8):e285-e290. doi: 10.1097/INF.0000000000004795. Pediatr Infect Dis J. 2025. PMID: 40073386
References
-
- Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American association for the study of liver diseases, American society of transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition[J]. Hepatology. 2014;60(1):362–15. doi: 10.1002/hep.27191 - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
