Food Additives: Emerging Detrimental Roles on Gut Health
- PMID: 40622070
- PMCID: PMC12232514
- DOI: 10.1096/fj.202500737R
Food Additives: Emerging Detrimental Roles on Gut Health
Abstract
Processed and ultra-processed foods have become dietary staples in many developed countries. A major constituent of these foods is a variety of synthetic chemical additives, which are used to improve the texture, preservation, and aesthetics of food. Evidence is mounting that synthetic chemicals used as food additives may have harmful impacts on health. Studies have linked certain additives to health conditions such as attention deficit hyperactivity disorder, cancer, and obesity. In addition, emerging evidence suggests that additives, such as emulsifiers, artificial sweeteners, colorants, and preservatives, may act as potential disruptors of intestinal homeostasis. Indeed, various studies have identified that food additives can impact gut health by modulating gut microbiota and intensifying intestinal inflammation. Considering the lack of known nutritional benefits of these additives and the accumulating evidence on the detrimental effects of these additives on gut health, further experimental, epidemiological, and clinical evaluations are imperative. This will provide significant advances in the prevention and management of gut health, including intestinal inflammation, and in enriching public knowledge on the harmful effects of these additives. In this review, we explore the effects of popular food additives on gut health with a particular focus on intestinal inflammation and examine the broader implications of these impacts on food safety policy and public health.
Keywords: food additives; food dye; food emulsifier; gut health; intestinal inflammation; microbiota; processed/ultra‐processed foods; public health.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


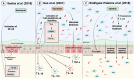


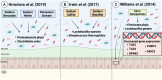

References
-
- Zhang K., Hornef M. W., and Dupont A., “The Intestinal Epithelium as Guardian of Gut Barrier Integrity,” Cellular Microbiology 17 (2015): 1561–1569. - PubMed
-
- Peterson L. W. and Artis D., “Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis,” Nature Reviews. Immunology 14 (2014): 141–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

