Mitochondrial Calcium Uniporter-Mediated Regulation of the SIRT3/GSK3β/β-Catenin Signaling Pathway in Vascular Remodeling
- PMID: 40622073
- PMCID: PMC12232586
- DOI: 10.1096/fj.202500369RR
Mitochondrial Calcium Uniporter-Mediated Regulation of the SIRT3/GSK3β/β-Catenin Signaling Pathway in Vascular Remodeling
Abstract
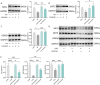
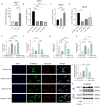
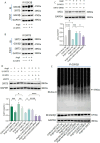
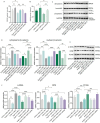
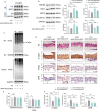
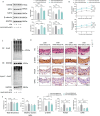
Calcium homeostasis plays a crucial role in regulating the phenotype of vascular smooth muscle cells (VSMCs) and vascular remodeling. This study aims to investigate the role of the mitochondrial calcium uniporter (MCU), which facilitates the uptake of Ca2+ into the mitochondria, in vascular remodeling and its underlying regulatory mechanisms. Vascular remodeling in rats was induced through either 21-day hindlimb unloading (HU) or 21-day angiotensin II (Ang II) infusion (0.7 mg/kg/day). Phenotypic switching of VSMCs and vascular remodeling were assessed. To induce phenotypic switching and clarify the underlying regulatory mechanisms, VSMCs were treated with Ang II (100 μmol/L). Gene manipulation was performed using plasmids, lentivirus, and adeno-associated virus serotype 9 (AAV9). Mitochondrial oxidative stress, Ca2+ distribution, and the expression of MCU, SIRT3, GSK3β, and β-catenin, along with GSK3β activity, SIRT3 ubiquitination, and GSK3β acetylation, were evaluated. The expression of MCU and SIRT3 in rat cerebral arteries was downregulated following HU and Ang II administration, which resulted in an increase in cytoplasmic Ca2+, a decrease in mitochondrial Ca2+, and a shift toward a synthetic phenotype in VSMCs. In vitro, Ang II treatment of VSMCs led to reduced expression of MCU, SIRT3, and GSK3β, and increased nuclear translocation of β-catenin. Knockdown of MCU caused an increase in cytoplasmic Ca2+ and a reduction in mitochondrial Ca2+, while MCU overexpression had the opposite effect, decreasing cytoplasmic Ca2+ and increasing mitochondrial Ca2+. Additionally, MCU overexpression decreased SIRT3 ubiquitination, mitochondrial oxidative stress, GSK3β acetylation, nuclear translocation of β-catenin, and VSMC phenotypic switching-these effects were blocked by SIRT3 knockdown. Moreover, MCU overexpression partially mitigated vascular remodeling in HU and hypertensive rats by inhibiting the GSK3β/β-catenin pathway and preserving SIRT3. Ang II regulates MCU protein expression, which is reduced in the HU and Ang II-induced hypertensive rat cerebral arteries. This reduction impairs cellular Ca2+ buffering and promotes mitochondrial oxidative stress. The stress response triggers the downstream degradation of SIRT3, which subsequently inhibits the activity of GSK3β via acetylation and promotes the nuclear translocation of β-catenin, thereby facilitating phenotypic switching and vascular remodeling.
Keywords: GSK3β/β‐catenin; SIRT3; angiotensin II; calcium; hindlimb unloading; microgravity; mitochondrial calcium uniporter; vascular remodeling.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Zhang L., “Region‐Specific Vascular Remodeling and Its Prevention by Artificial Gravity in Weightless Environment,” European Journal of Applied Physiology 113 (2013): 2873–2895. - PubMed
-
- Xie M., Zhang L., Ma J., and Cheng H., “Functional Alterations in Cerebrovascular K+ and Ca2+ Channels Are Comparable Between Simulated Microgravity Rat and SHR,” American Journal of Physiology. Heart and Circulatory Physiology 289 (2005): H1265–H1276. - PubMed
-
- Zhang R., Bai Y. G., Lin L. J., et al., “Blockade of AT1 Receptor Partially Restores Vasoreactivity, NOS Expression, and Superoxide Levels in Cerebral and Carotid Arteries of Hindlimb Unweighting Rats,” Journal of Applied Physiology 106 (2009): 251–258. - PubMed
-
- Zhang R., Jiang M., Zhang J., et al., “Regulation of the Cerebrovascular Smooth Muscle Cell Phenotype by Mitochondrial Oxidative Injury and Endoplasmic Reticulum Stress in Simulated Microgravity Rats via the PERK‐eIF2α‐ATF4‐CHOP Pathway,” Biochimica et Biophysica Acta 1866 (2020): 165799. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

